Although breast magnetic resonance (MR) imaging was initially hindered by the limited availability of coils, protocols, and particularly breast biopsy devices, standardized imaging and specialized equipment necessary to perform high-quality MR imaging of the breast is now readily available. Those performing breast MR imaging should also be capable of performing biopsies, or at least have a close association with a facility that does. Optimal management of patients requires a good understanding of the indications and technique of breast MR imaging biopsies. In this article the indications for breast MR interventional procedures along with the techniques used are described. In addition, the histologic process encountered and recommended strategies for patient management are discussed.
The use of magnetic resonance (MR) imaging of the breast has increased dramatically in the last few years. Breast MR imaging offers a unique imaging technique for which many defined indications proven to benefit certain subsets of patients exist. MR imaging provides information not available through other breast imaging modalities routinely used at present. The unique qualities of breast MR imaging, including lack of ionizing radiation or limitation by breast density, make it particularly beneficial to young and high-risk patients. Although breast MR imaging was initially hindered by the limited availability of coils, protocols, and particularly breast biopsy devices, standardized imaging and specialized equipment necessary to perform high-quality MR imaging of the breast is now readily available.
Given the unique properties of this modality, familiarity with breast MR imaging is necessary for all radiologists interpreting breast imaging. It is encouraged that those performing breast MR imaging also be capable of performing biopsies, or at least have a close association with a facility that does. Familiarity with the indications and technique of breast MR imaging biopsies is recommended for all, regardless of whether they actively perform biopsies. Optimal management of patients requires a good understanding of these processes. In this article the indications for breast MR interventional procedures along with the techniques used are described. In addition, the histologic process encountered and recommended strategies for patient management are discussed.
Indications for interventional MR
MR interventional procedures include both wire localization and needle biopsy. Historically, wire localization procedures were the first to be employed, as MR-compatible needle/wire sets became available. When vacuum-assisted biopsy devices were later developed for use in magnets, this procedure quickly replaced the majority of wire localizations. As with lesions found by palpation or other breast imaging modalities, suspicious lesions identified by MR are preferentially sampled percutaneously rather than surgically. With routine use of postbiopsy markers, sampled lesions requiring subsequent excision can easily be localized either mammographically or sonographically, thus reducing the need and indications for MR-guided wire localization. Therefore, the indications for core biopsy in general are discussed first.
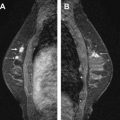
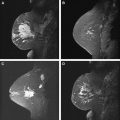
Studies have shown that MR imaging has a high sensitivity for the detection of invasive carcinoma of the breast. With the higher resolution scanning now achieved with dedicated breast coils along with advances in interpretation criteria, ductal carcinoma in situ (DCIS) is being detected more frequently. Lesions detected on MR imaging include enhancing masses and nonmass-like enhancement. Both can indicate malignancy and, depending on the morphology and kinetics of individual lesions along with the clinical scenario, may require biopsy. Detection of malignant disease in another quadrant to an ipsilateral cancer or in the contralateral breast can have a profound effect on surgical management ( Fig. 1 ). Some MR-detected lesions will prove to be detectable by mammography or sonography, particularly what has been termed “second-look” ultrasound (US), but many will not. For those suspicious MR-detected lesions not identified on mammography or sonography, MR-guided biopsy is indicated.

Correlation with Other Imaging Studies
Correlation with other imaging studies is the first step in management of MR-detected lesions that are suspicious enough to require biopsy. Recent mammograms should be reviewed to assess for a mammographic correlate. Sometimes, a small enhancing mass on MR can be easily determined to represent a stable mass such as a fibroadenoma or an intramammary lymph node seen on mammography. If no mammograms are available for correlation, diagnostic mammography should be performed, with particular attention to the area(s) of interest. The majority of additional MR-detected lesions, however, will usually prove not to be identified on mammography.
Focused US is probably more useful than mammography in the detection of MR-detected lesions. If a sonographic correlate is found, then biopsy is facilitated. US-guided percutaneous biopsy can be performed quicker, easier, at lower cost, and with more patient comfort than MR-guided biopsy. Second-look US has been shown to detect approximately one-quarter to one-third of MR lesions. Detection of mass lesions is higher than that of nonmass lesions, which is not surprising given that mass lesions are more likely to represent invasive disease and nonmass lesions in situ disease. DCIS is, of course, more difficult to detect with US. Of note, the malignancy rate has been found to be higher if a US correlate is found. However, lesions without a US correlate still have a substantial rate of malignancy such that biopsy is still necessary.
Correlation of MR-detected findings with other imaging modalities can be challenging because of the differences in patient positioning during mammography, US, and MR. Mammography is performed with the patient upright and the breast in firm compression, US with the patient supine and the breast tissues uncompressed and flattened dependently against the chest wall, and MR with the patient prone and the breasts hanging pendulously. Correlating a lesion seen on one modality with another can be challenging. It is common among interpreters of breast imaging studies to describe the distance of a lesion from the nipple. For other than the smallest breasts, this distance may change substantially among the different imaging studies because of the markedly different imaging positions. Localization of a lesion within the anterior, mid, or posterior thirds of the breast is usually adequate. Otherwise, conforming to strict nipple-lesion distances may result in markedly inaccurate areas of the evaluated breast. In particular, when performing US for MR lesions, a generous range of tissue area around the site of the measurement from the nipple is recommended as these 2 study methods have the greatest degree of differences in breast position. Use of landmarks such as cysts or other benign masses may help increase detection of MR lesions. Assessment of the glandular tissue and fat planes can also be helpful.
Confidence that a US-detected lesion correlates with the MR lesion of interest is always somewhat problematic. The differences in breast position as outlined above constitute a main reason. Detection of the smaller masses and nonmass-like enhancement by US will require sampling of more subtle sonographic lesions. Confidence that the “correct” area has been sampled will sometimes be uncertain. Studies have shown that higher rates of falsely benign findings occur with such biopsies, indicating that the MR-detected lesion was not correctly sampled. A study by Meissnitzer and colleagues showed that 10 of 80 lesions that underwent US biopsy with what was considered a benign, concordant result on follow-up imaging was found not to correlate with the MR finding. On subsequent biopsy, 5 of 9 lesions were shown to be malignant. This result underscores the difficulty in correlating the 2 imaging studies. If there is a reasonable degree of uncertainty in the sonographic findings, biopsy under MR guidance may be a better option. If US biopsy is performed, then careful review of the histologic findings to assess for concordance is required and follow-up MR is also necessary.
Attempts at improving correlation of MR and US imaging are underway, with modification of coil design along with development of software, aiming at a greater degree of accuracy in finding lesions and confidence in assessing concordance. This upgrade should greatly improve the chance of finding lesions, increase the confidence in targeting the correct lesion, and reduce the need for follow-up MR examinations.
Core Biopsy
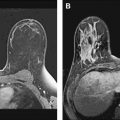
As stated earlier, core biopsy is the primary procedure performed under MR guidance. Wire localizations, however, may be necessary under certain circumstances. In a patient with ipsilateral newly diagnosed cancer, bracketing of areas of MR enhancement suspicious for extent of disease around the index cancer may be appropriate through MR-guided preoperative wire localization. For example, this would include areas in the same vicinity as the primary cancer not sufficiently separate to require preoperative core biopsy if management would not have been changed. Whereas technical considerations are discussed below, limitations for prone MR vacuum-assisted biopsy are similar to prone stereotactic procedures and include lesions that are far posterior or in the axillary tail as well as lesions near the nipple ( Fig. 2 ). For such lesions, wire localization may be technically possible whereas vacuum-assisted biopsy would be difficult or impossible. Also, wire location rather than the larger gauge vacuum-assisted biopsy may be preferred for some superficial lesions or lesions adjacent to implants.

MR-guided biopsy technique
Although it may seem intimidating to some at first, MR-guided biopsies are actually easily learned and performed. Other than the obvious differences in imaging used, the technique of patient positioning and lesion sampling with vacuum-assisted devices is markedly similar to stereotactic biopsy. Thus most radiologists performing breast biopsies will be familiar and become quickly comfortable with the technical aspects of the procedure. Lesion detection and targeting can be the most challenging part of the procedure.
Imaging and Report Review
Good quality prebiopsy MR imaging and interpretation is necessary. If imaging quality is different, particularly when performing biopsies from outside facilities, repeat MR imaging prior to biopsy may be indicated. However, given the cost of these imaging studies along with the additional time involved to repeat the study, sometimes scheduling the patient for biopsy is more appropriate than repeating a diagnostic MR examination which, if still suspicious, would require the patient to return yet again.
Review of the preprocedure imaging and reports is necessary before starting the procedure. For cases performed at another facility, importing the study into a PACS (picture archiveing and communications system) is advised such that images are readily available for reference during the procedure. Importing the prior study into PACS permits linking of various images in various planes, particularly sagittal and axial, which is necessary for confidence in targeting during biopsy. Such a viewpoint is not always possible when viewing images directly from a disc.
Review of the prior studies is also necessary to ensure sampling of the correct lesion. Ideally, such studies should include annotated images and/or image slice numbers detailed in the report to avoid uncertainty for the performing radiologist at the time of the procedure. Also, review of the prior images allows planning when biopsy of more than one site is necessary. Whereas sampling of more than one site in the same breast is relatively easy, bilateral biopsy requires sequential sampling. Although this process is relatively quick with the coils and imaging protocols available at present, targeting and sampling of the most suspicious lesion or breast first is recommended, particularly if lesions have washout kinetics. Having a clear plan before starting the procedure can allow the technologist to image in the shortest amount of time.
Canceling or Aborting the Procedure
It is well understood that many lesions for which MR biopsy is recommended are ultimately not reproduced, thus resulting in clinicians aborting or canceling the procedure. This situation occurs because there is considerable overlap in the MR appearance of normal breast glandular enhancement and other benign findings with malignant processes. Often such subtle findings, particularly nonmass-like enhancement, may represent “hormonal” breast glandular tissue and thus may not be reproduced at the time of biopsy. Other factors may include the amount of compression used, which may be slightly greater during biopsy than routine breast MR imaging, given that the fenestrated grid immobilizes the breast. Too much compression can inhibit intravascular contrast, thus decreasing lesion conspicuity. In a study by Hefler and colleagues, repeat imaging was performed in 29 patients in whom MR biopsy had been canceled due to lack of enhancement. Although most of these (25/29) did not enhance on follow-up, 4 of 29 did persist and 3 of these (75%) proved to be malignant. For reasons like this, the grid should be placed firmly enough against the breast to restrict breast movement, but without being too firm to be uncomfortable for the patient or limit blood flow. If a previously noted lesion is not seen during biopsy, slight release of compression and repeat imaging should be performed before canceling the procedure. Reinjection of additional contrast if a period of time has elapsed could be considered if doing sequential breast biopsies. The efficacy of this technique, however, has not been proven in the literature. It is possible that malignant lesions could not be evident on reinjection, being obscured due to background enhancement.
T1 Imaging
After positioning the patient as comfortably as possible in the breast coil with the breast immobilized, nipple straight, and no skin folds present, T1-weighted fat-saturated sequences in the sagittal projection are generally performed. If isotropic imaging is available, software can permit ease of viewing information in multiple planes on the workstation. A precontrast sequence is recommended to check for good imaging parameters, such as fat saturation, as well as the appropriate field of view. Also, precontrast images will prove useful if subtraction images are required to detect subtle enhancing areas.
Targeting the Lesion
After the area of interest is identified, targeting the lesion simply depends on identifying the correct opening within the grid and deciding into which hole in the needle guide the probe will be inserted. Most MR procedures are approached from the lateral, or less commonly the medial direction. If a cranial approach is available, similar steps can be taken with imaging in the axial projection. If targeting is done manually on the console or workstation, scrolling through the images in the sagittal plane with a cursor placed over the lesion to the skin/grid will provide the depth information. The distance from the skin to lesion can be determined on the monitor as the difference of the slice numbers and the thickness of each slice. For example, if slice thickness is 3 mm and there are 10 images from skin to lesion, then the depth is 30 mm, or 3 cm.
For core biopsy, noting the X and Y coordinates (established as the designated hole in the needle guide) and the Z depth (determined as skin to lesion distance) is all that is necessary to initiate the biopsy. The extra depth to account for the thickness of the needle guide block (2 cm) is already added into the depth markings on the introducer sheath. In other words, the 0 position actually starts at 2 cm from the tip of the sheath, rather than the distal end.
Incision and Insertion for Vacuum-Assisted Core Biopsy
After local anesthetic is delivered both as a skin wheal and to deeper tissues along the projected needle track, a small slit in the skin is made with a scalpel. The trocar is then placed through the sheath and both are inserted together through the needle guide to the predetermined depth in the breast. Use of a slight rotation motion of the trocar facilitates penetration of the tissues with the least amount of displacement. Once inserted, the trocar is then withdrawn from the sheath and replaced with a plastic obturator for MR imaging. If using a ceramic probe (Mammotome; Ethicon Endosurgery, Cincinnati, OH, USA), which allows imaging of the actual sampling probe rather than an obturator, this will be inserted beyond the Z=0 position such that the lesion sits within the middle of the sampling trough. MR imaging is then performed to document accurate needle position in all directions. As vacuum-assisted biopsy is directional, small offsets of the lesion in the X and Y directions can be accommodated by preferential sampling in the desired direction. If the distal end of the obturator is not at the desired depth, it can be corrected by either withdrawing or advancing the sheath with the trocar reinserted.
Sampling
Sampling is quickly and easily performed with vacuum-assisted techniques. In general, circumferential sampling is performed although, as mentioned earlier, directional sampling can be performed if the lesion predominantly resides in a particular direction. Most vacuum-assisted devices used for MR are either 9- or 10-gauge. Six to 12 core samples generally are felt to be adequate for diagnostic purposes.
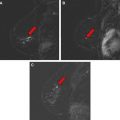
Repeat MR imaging is again performed to assess for completeness of sampling. Documenting adequate sampling on MR biopsy can be challenging. Visualization of small enhancing lesions can be very difficult because of the combination of local anesthetic, hemorrhage, saline, and air all present at the biopsy site ( Fig. 3 ). If the enhancing lesion is felt to be inadequately sampled, the probe can be reinserted and additional samples can be obtained.

After sampling is complete, the biopsy cavity is irrigated (if using saline) and aspirated before probe removal. A biopsy marker is then deployed at the site. A final set of images is obtained to document accurate marker deployment. The metallic artifact generated by the marker clip can be difficult to differentiate from air and other biopsy site changes on fat-saturated images. Nonfat-saturated T1-weighted images may permit better visualization of the metallic clip, so this may be preferred as the last sequence.
MR-Guided Needle Localization Core Biopsy
MR-guided needle localizations are performed slightly differently than vacuum-assisted core biopsies. An MR-compatible 20-gauge hookneedle is used (Cook, Bloomington, IN, USA). This needle has centimeter markings on its length; however, unlike the sheath used for core biopsies, the thickness of the needle guide is not accounted for. Therefore, after determining lesion depth, as described above, an extra 2 cm has to be added to the depth. In addition, as with all needle localization performed with a Kopans needle, the lesion should ultimately reside within the thick portion of the wire and not the tip; therefore, an additional increment of 5 to 10 mm depth must also be added.
It is important to keep in mind that, unlike mammographically-guided wire localizations, the wire is deployed through the needle in the orthogonal projection to how it was inserted, the needle is inserted, and wire deployed all in one direction, with the breast in compression throughout. Therefore, depending on the compressibility of the breast and corresponding amount of accordion effect, the distance beyond the lesion to which the needle should be inserted varies. In general, in a fatty compressible breast the accordion effect is greater, therefore a distance of 5 mm may be adequate. In dense, less compressible tissues, a distance of 1 cm is reasonable. After documentation of accurate needle position and wire exchange, the compression of the breast may be released slightly before the last imaging series such that a better assessment of the final wire position can be made. Of course, when the patient is removed from the magnet and resumes the upright position, the wire may migrate from its position during imaging.
The wires used for MR-guided localizations are somewhat more fragile than their counterparts used in mammography or sonography, and breakage or fragmentation has been reported. It is prudent to inform the surgeons of this fact, such that extra care may be taken in the operating room to avoid accidental breakage or fracture of the wire.
Software for MR-Guided Procedures
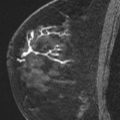
Software systems providing computer-aided detection for MR are available and commonly used in breast MR interpretation. These systems also provide targeting and tools for MR-guided procedures that greatly facilitate the biopsy process. These tools include visual guides as to precise needle insertion points and depth determination, and visual representation of the needle position in relation to the lesion ( Fig. 4 ). Such programs even include warnings when there is not adequate breast thickness for appropriate needle position or trough placement. Even if such programs are routinely used, knowledge of the manual steps involved is advised to facilitate trouble-shooting or permit successful biopsy if the system is not functioning for any reason.