Fig. 10.1
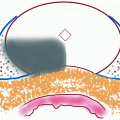
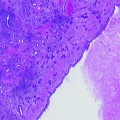
Four cases of prostate cancers identified at multiparametric 1.5 T MRI and diagnosed by targeted biopsies. Axial T2-W, DW, T1-W DCE, and axial TRUS images. Visual reading of all sequences allows suspicious score and location determination. Suspicious areas at MRI are shown with red arrows. Case #1: cancer located in PZ at midline with 7 mm hyposignal area (T2-W), low ADC (DW), and intense early enhancement (T1-W DCE) with a suspicious score of 5. TRUS showed hypoechoic corresponding area. PSA = 7.9 ng/ml. Maximal cancer length was 6 mm at targeted biopsies Gleason score 4 + 4. Systematic biopsies were negative. Case #2: cancer located in the left lobe of PZ with 10 mm hyposignal area (T2-W), low ADC (DW), and intense early enhancement (T1-W DCE) with a suspicious score of 5. TRUS showed hypoechoic corresponding area. PSA = 6.9 ng/ml. Maximal cancer length was 6 mm at targeted biopsies Gleason score 4 + 4. Systematic biopsies were negative. Case #3: cancer located in right anterolateral horn of PZ with 19 mm hyposignal area (T2-W), low ADC (DW), and weak enhancement (T1-W DCE) with a suspicious score of 4. TRUS showed equivocal hypoechoic corresponding area. PSA = 7.8 ng/ml. Maximal cancer length was 4 mm at targeted biopsies Gleason score 3 + 4. Systematic biopsies were negative. Case #4: APC located in AFMS anterior to right TZ at apex with 13 mm hyposignal area (T2-W), low ADC (DW), and intense early enhancement (T1-W DCE) with a suspicious score of 5. TRUS showed equivocal hypoechoic corresponding area. Maximal cancer length was 7 mm at targeted biopsies and systematic biopsies were negative
Nevertheless, the lack of standardization in the conduct, reporting, and evaluation of MRI sequences has notably led to discrepancy in its acceptance and use. Therefore, a recent European MRI Consensus Panel recommended that this modern imaging modality needs to be delivered in a quality controlled manner with uniformly high standards and incorporated as a test prior to biopsy [6]. The panel reached agreement on 67% of 260 items related to imaging sequence parameters. For instance, T2-weighted, dynamic contrast-enhanced, and diffusion-weighted MRI were the key sequences incorporated into the minimum requirements but spectroscopy was not recommended. Consensus was also reached on 54% of 260 items related to image interpretation and reporting, including features of malignancy on individual sequences. A five-point scale was agreed on for communicating the probability of malignancy, with a minimum of 16–27 prostatic sectors of analysis to include a pictorial representation of suspicious foci (Fig. 10.2). Due to the increase in signal-to-noise ratio, 3 T MRI clearly improves spatiotemporal and spectral resolutions of prostate imaging on all sequences. However, imaging criteria for malignancy seem to be comparable at 3 and 1.5 T with pelvic coil. A pelvic phased array coil for 1.5 T MRI was deemed sufficient for standard clinical practice and endorectal coil is not mandatory anymore for prostate cancer detection.
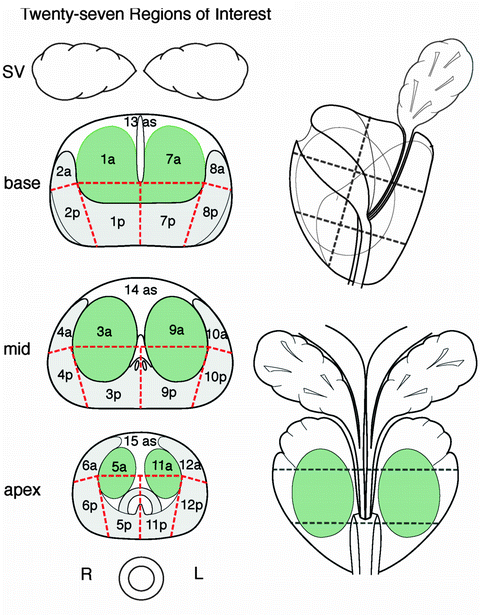

Fig. 10.2
Twenty-seven-sector standardized MRI report prostate scheme. Average axial sections at prostate base, mid-gland, and apex are subdivided into four posterior regions (p) (mid-lobar and lateral), four anterior regions (a) (mid-lobar and lateral), and three anterior stroma regions (as), at the center, anterior to glandular zones. Twelve-core biopsy scheme samples the 12 posterior sectors [16]. Adapted from Dickinson et al. [6]
Techniques of Targeting Biopsies Guidance to an MRI-Suspicious Area
Targeting biopsies to an MRI-suspicious area can be carried out in several ways such as free-hand “cognitive” targeted biopsy under TRUS guidance, MRI-directed real-time biopsy (MRI guidance) or real-time virtual navigation system with fusion of the MR-images to the ultrasound images, and targeted biopsy under TRUS/MRI fusion guidance.
Free-hand “cognitive” targeted biopsy under TRUS guidance is easy to perform as office-based procedure by the urologist. There is indeed a fundamental methodological limitation of the biopsy technique to use virtual MR-data for TRUS-guided biopsy, in which the biopsy is not truly MR guided. The accuracy of TRUS-guided biopsy with cognitive virtual use of MR-detected suspicious findings has not been formally studied. However, there are convincing results that additional two cores from operator use of MR-data for TRUS-guided free-hand cognitive biopsy accurately hit the lesion. Hence, the correlation coefficient between the cancer length on targeted biopsies (median 8 mm, IQR: 7–12) and the antero-posterior diameter of the largest area suspicious for malignancy on MRI (median 10 mm, IQR: 8–14) was r 2 = 0.6 (p < 0.001) [12]. In this study, MRI was performed immediately before each biopsy. All the patients underwent 12 systematic TRUS-guided biopsies and for each area of suspicion (anterior or posterior gland), 2–4 additional targeted biopsy cores were obtained (Fig. 10.1), resulting in a total mean of 15 (range 14–18) biopsy cores per patient. Each MRI scheme was evaluated simultaneously by both a radiologist and an urologist which allowed the urologist performing the biopsies to localize the suspicious areas during TRUS. Targeted biopsies were performed by free-hand, sampling each one of the suspicious areas based on the 27-section MRI scheme (Fig. 10.2). Suspicious posterior areas at MRI are mostly associated with corresponding hypoechoic areas at TRUS, which help needle guidance. Suspicious anterior areas on TRUS are not identified in all cases due to the heterogeneity of the transition zone at TRUS and the normal hypoechogenicity of the AFMS. However, in some cases, the anterior area corresponding to the MRI abnormality shows equivocal hypoechoic areas with contours roughly similar to the contours seen at MRI. These TRUS anterior abnormalities are used as well as anatomic landmarks to target the biopsies despite their (very) low suspicious pattern in all cases and the fact that they would not have even been identified without pre-biopsy MRI.
MRI-directed real-time biopsy (MRI guidance) was also proven to be very effective in improving detection [13–18]. In these studies of patients with history of previous negative biopsy series, the detection rates with MRI guidance were 59, 30, 52, 41, 45.5, and 31.5%, respectively. Classically, the patient must first be examined in a closed MR scanner and then biopsied in an open scanner because open MR scanners do not provide optimal tissue contrast [19]. However, MR-guided biopsy can also be performed in a closed system with special equipment. In the study by Hambrock et al., 68 consecutive patients with two or more previous biopsy series and persistent suspicion of prostate cancer were enrolled [13]. Using a median of four MR-guided biopsies, the cancer detection rate was 56% and was significantly higher than that of matched TRUS-guided prostate biopsy population (p = 0.01). In that study, patients underwent 3 T MR-guided biopsy after an average of 2 weeks (range 1–6) from initial MRI to localize possible tumors, which is cumbersome to achieve routinely. MR-compatible robotic systems for biopsy guidance are under evaluation. This technique improves the accuracy of lesions sampling [20]. However, in-bore MRI guidance requires specialist expertise, additional equipment, and is lengthy and expensive. No comparative study of various targeting modalities is available.
The concept of fusion of the MR images to the ultrasound images used at the time of biopsy, using virtual navigation systems with rigid registration or 3D acquisition with elastic registration, is under evaluation. In one study, this fusion of real-time TRUS and prior MR images of the prostate proved its feasibility and enables MRI-guided biopsies outside of the MRI suite [21]. In a recent study by Pinto et al., 101 patients with a median PSA of 5.8 ng/ml underwent 3 T Mp-MRI, 12-core TRUS-guided systematic biopsy, and MRI/US fusion-guided biopsy at the same setting. With an average of 5.8 cores taken during MRI/US fusion, the overall cancer detection rate was 54.4% and was significantly correlated with suspicion of cancer on MRI (27.9% for low, 66.7% for moderate, and 89.5% for high suspicion). In the same study, MRI/US fusion-guided biopsy detected more cancer per core than standard 12-core TRUS biopsy. However, no specific information was provided by the authors in regard to biopsy location and quality of sampling (cancer length and grade in each positive core).
Role of Pre-biopsy MRI with Targeted Biopsies
Pre-biopsy MRI with targeted biopsies improves detection accuracy, allows cancer upgrading and upstaging, and may be used as a triage test to rule out cancer and avoid biopsies.
The current diagnostic process, i.e., the use of TRUS guidance to take 10–12 transrectal needle biopsies from different parts of the prostate in a systematic fashion, has inherent random and systematic errors related to the biopsy technique, and underestimates the true cancer grade in up to one-third and the cancer burden in up to one half of men diagnosed with low-risk disease [22]. On the whole, TRUS is used to locate the prostate gland itself, but otherwise plays little part in guiding the biopsy procedure. In a series of 544 patients with suspicion of prostate cancer, sensitivity and specificity of TRUS for identification of prostate cancer were 41 and 85%, respectively [23]. The PPV was as low as 53%. As a result of these errors, localization of individual tumors within the prostate is poor, whereas Mp-MRI is very sensitive for both anterior and posterior cancer detection (Fig. 10.3); recent published data support the concept of MRI-targeted biopsy [9, 24].
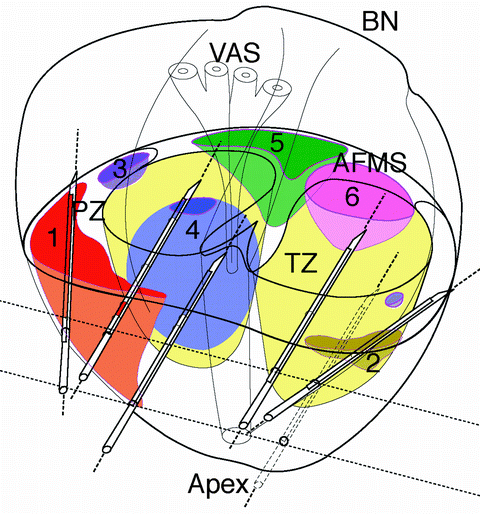

Fig. 10.3
3D postero-lateral view of a schematic prostate gland showing zonal anatomy. PZ peripheral zone, TZ transition zone, AFMS anterior fibromuscular stroma. Average cancers size and location are displayed. Posterior systematic biopsy needles and tracks are displayed (lateral and medio-lobar at mid-gland and medio-lobar at apex). (1) PZ postero-lateral cancer 4 cc sampled by both lateral and mid-lobar biopsies. (2) PZ postero-lateral cancer 0.9 cc sampled by lateral biopsy. (3) PZ antero-lateral cancer 0.6 cc not sampled by posterior systematic biopsies. (4) TZ anterior cancer 2 cc sampled by mid-lobar biopsies. (5) AFMS anterior cancer 1 cc not sampled by posterior systematic biopsies. (6) TZ/AFMS anterior cancer 1 cc not sampled by posterior systematic biopsies. Adapted from Bouye et al. and Haffner et al. [29, 30]
In a retrospective series of 555 men referred for elevated PSA, targeted biopsies to lesion identified at pre-biopsy MRI were compared to systematic biopsies [25




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree