Radiography:
Early: deep soft tissue swelling
Mid: cortical fraying
Late: moth eaten
MRI: positive 2 days after symptoms
Overestimates extent
T1 wide zone of transition
Cortical destruction and periosteal elevation
Intraosseous, subperiosteal, or soft tissue abscesses/edema
1.
In most cases, organisms initially target the highly vascular metaphysis. Sluggish blood flow in the large, venous sinusoidal loops in this area allows bacteria to adhere to endothelium and penetrate capillaries to enter the extravascular space. Limited phagocytic activity in metaphyseal capillaries facilitates bacterial proliferation. Intraosseous pressure increases due to the inflammatory response and exudate. Edema and vascular congestion progress to small vessel thrombosis, bone necrosis, and bone resorption. Subsequent spread of disease varies with patient age, as discussed below (Fig. 19.1).
2.
In infancy (up to about 18 months), blood vessels penetrate the physis to extend from the metaphysis to the epiphysis. This allows infection to spread readily from the metaphysis to the epiphysis (Fig. 19.2). Septic arthritis is also very common, as infection can spread directly to the joint either by extension through the epiphysis or by perforating the metaphyseal cortex (which is intra-articular in the hip, shoulder, and elbow). Conversely, infection that begins within the joint may infrequently spread to the epiphysis and then the metaphysis. Avascular necrosis of the epiphysis is very common, as high pressure within the joint restricts blood flow. Septic arthritis of the hip frequently results in avascular necrosis of the femoral head, with eventual growth disturbance.
3.
During childhood, contrasted with infancy, vessels do not penetrate across the physis, and thus the physis serves as a barrier to transphyseal spread to the epiphysis. Infection is initially confined to the metaphysis, but as intramedullary pressure increases, pus eventually penetrates the normal metaphyseal fenestrations to spread via Haversian and Volkmann canals. After reaching the subperiosteal space, it readily dissects along the diaphysis, as the periosteum is relatively loosely attached to bone in children. Subperiosteal abscesses may form. In addition, infection may penetrate the periosteum and spread into the soft tissues. Alternatively, septic arthritis may develop at joints with an intra-articular metaphysis.
4.
After closure of the growth plate, osteomyelitis behaves as it does in adults. The metaphysis is still the most common location (due to its vascularity and the slow flow of blood in sinusoids), but epiphyseal spread is common, as vessels again cross the growth plate.
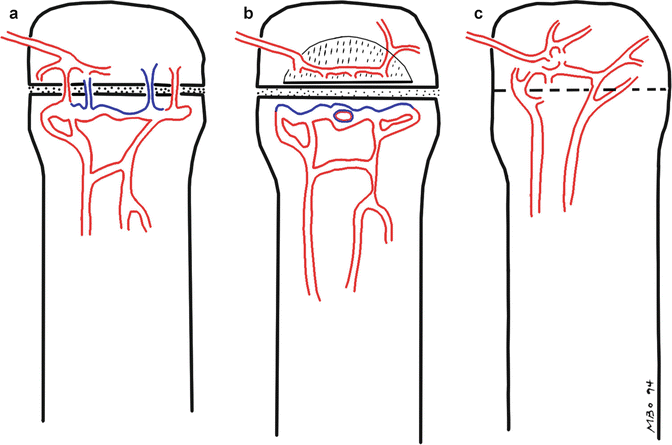
Fig. 19.1
The three stages of vascular development of the growth plate. (a) Until approximately 18 months, blood vessels from the epiphysis and the metaphysis cross the physis, allowing infection to easily spread. (b) From about 18 months until the physes close, the physis serves as a barrier to infection, since blood vessels do not traverse this area. Metaphyseal vessels terminate in sinusoids, where blood flow is sluggish. (c) After physeal closure, infection again spreads readily between the epiphysis and the metaphysis, via blood vessels that cross the physis as the bony scar is resorbed. (Red arterial supply, Blue venous drainage)
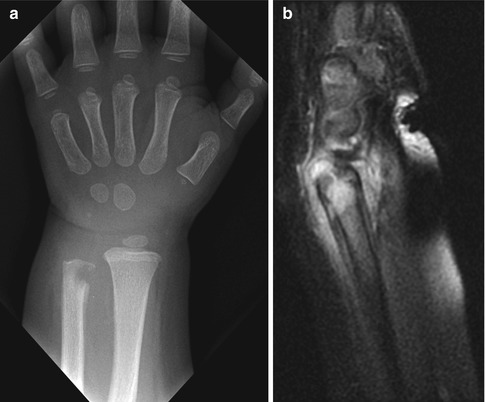
Fig. 19.2
Metaphyseal osteomyelitis in a 16-month-old. (a) Lytic defect in the distal ulna with very early periosteal reaction and extensive para-ulnar soft tissue swelling. (b) Sagittal T1-weighted (T1-W) gadolinium-enhanced fat-suppressed (FS) MRI shows a tract crossing the physis, with epiphyseal enhancement. Abnormal signal extends into the joint
1.2 Subacute Osteomyelitis (Box 19.2)
Box 19.2 Brodie Abscess
Eccentric cortical or medullary abscess of subacute osteomyelitis |
Often drains to physis |
Radiography: central lucent focus, surrounding sclerosis, draining channel |
Mild periosteal reaction, localized |
MRI: penumbra |
Subacute osteomyelitis develops if the host response or incomplete antibiotic therapy partially suppresses the infection or if the pathogen is indolent. Presentation is more insidious, with symptoms present for 2 weeks or longer [6]. Instead of the exuberant inflammatory process often encountered in acute osteomyelitis, the infection is more localized, usually confined to the metaphysis, and shows little periosteal thickening. The lower extremity is almost invariably involved, with over 90 % of lesions in the femur or tibia. Over 60 % are metaphyseal, and most occur at the distal tibia. Subacute osteomyelitis may occur in the epiphysis. Most common in adolescents and males, subacute osteomyelitis has not been described in patients younger than 6 years of age.
Subacute osteomyelitis may manifest as a Brodie abscess, which develops in the cortex or medulla and is usually eccentric. It usually drains to the physis and may extend to the epiphysis. Eburnated bone along with fibrous and granulation tissue encloses a central area of osseous necrosis. Staphylococcus is the usual pathogen [7].
1.3 Chronic Osteomyelitis (Box 19.3)
Box 19.3 Chronic Osteomyelitis
Involucrum: thick, ossified periosteal reaction |
Sequestrum: central sclerotic infarcted bone |
Cloaca: tract penetrating cortex, +/− to skin |
Chronic osteomyelitis has been present for at least 6 weeks and is more common in developing nations. Periosteal thickening has typically progressed, thickened, and ossified, forming an involucrum (from the Latin, involvere, envelop) that isolates the more central infection. Meanwhile, increasing pressure causes cortical bone infarction and necrosis, forming a sequestrum, which may then serve as a nidus of infection. The central infection may break through the cortex or penetrate to the physis, forming a (from the Latin, cloaca, sewer), which then permits the inflammatory debris to drain to the skin via a sinus tract [7]. Formation of an involucrum and sequestrum is unusual if antibiotics are administered at an early stage.
Erythrocyte sedimentation rate and white blood cell count can be normal and also nonspecific. Blood cultures are positive in up to 60 % of cases, whereas cultures of aspirated material yield an organism in as many as 85 % [7].
1.4 Imaging
Radiographs
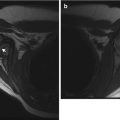
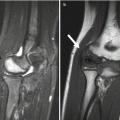
Radiographs are the initial imaging modality for acute osteomyelitis. While they help exclude such entities as tumors and fractures, they are often normal in cases of acute osteomyelitis. Deep soft tissue swelling may be apparent after 2 days of symptoms (Fig. 19.3), but bony findings are not evident for 1–2 weeks in the long bones and often much longer in the pelvis or spine. Demineralization of 30–50 % is necessary before bone lysis becomes apparent, but subtle cortical fraying due to subperiosteal resorption may be seen earlier (Fig. 19.4). Demineralization is usually due to disuse and confers a worse prognosis. In the appropriate clinical setting, osteolysis manifesting with a permeative or moth-eaten appearance, accompanied with periosteal reaction and surrounding subtle sclerosis, may allow diagnosis (Fig. 19.5). Physeal widening and epiphyseal destruction may occur in infants.
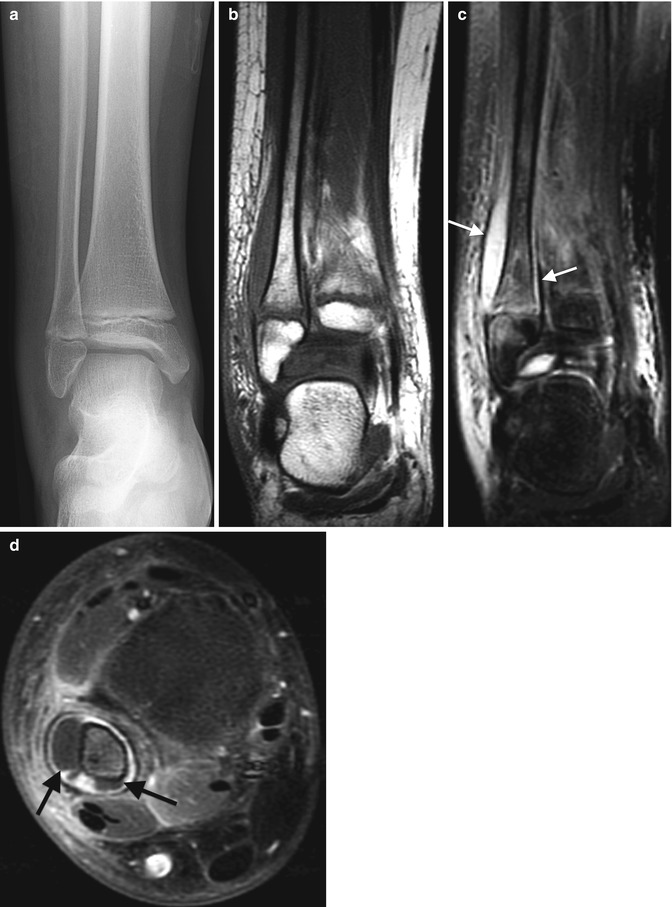
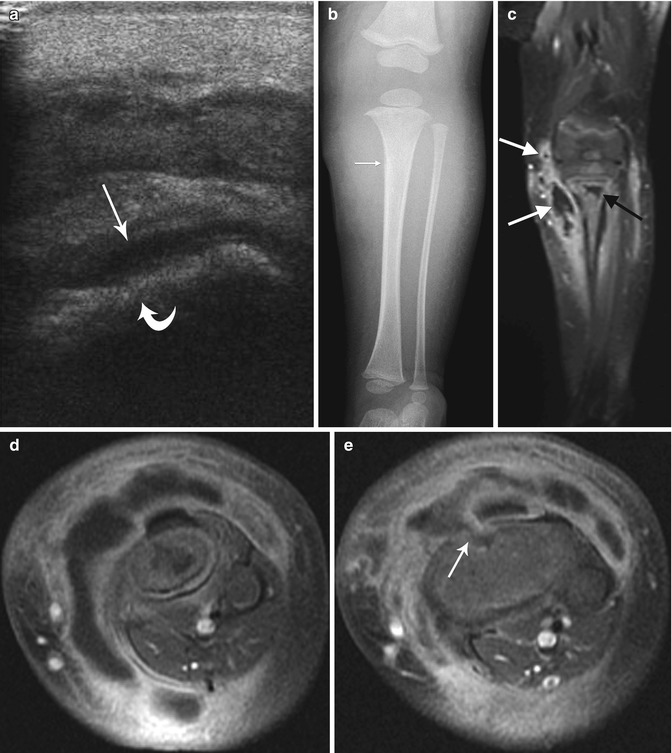
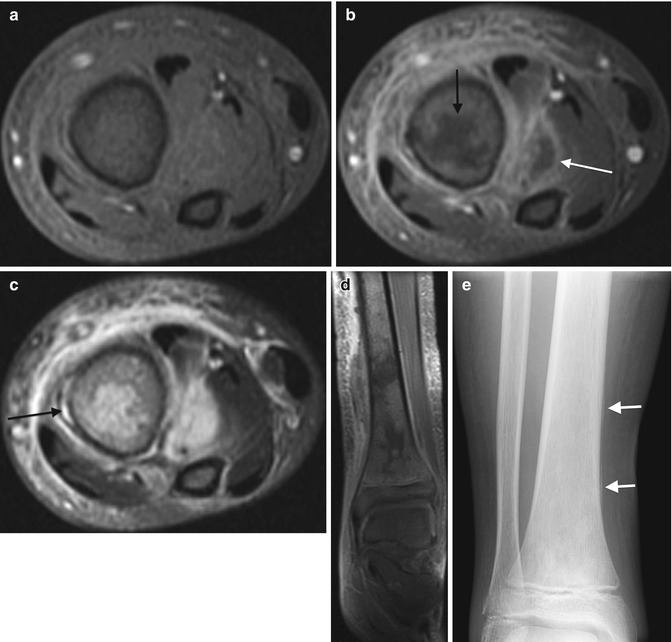

Fig. 19.3
Acute Staphylococcus aureus osteomyelitis with subperiosteal abscess. (a) Marked lateral soft tissue swelling and loss of subcutaneous fascial planes. (b) Contemporaneous T1-W coronal MRI shows periosteal thickening. (c) Coronal T2-weighted (T2-W) FS MRI shows subperiosteal fluid (arrows) with edema of the distal fibular metaphysis and epiphysis. (d) Gadolinium-enhanced FS T1-W axial image delineates subperiosteal abscesses (arrows) along the fibula

Fig. 19.4
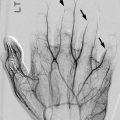
Staphylococcus aureus osteomyelitis with subperiosteal, soft tissue, and intramedullary abscess formation. (a) Initial sagittal US shows cortical irregularity (curved arrow) along with subperiosteal fluid (arrow). (b) Four days later, proximal tibial bone density is slightly decreased, and there is a thin line of periosteal reaction (arrow). Soft tissues are edematous. (c) Coronal T1-W FS gadolinium-enhanced MRI shows marrow enhancement with intramedullary abscess (black arrow) and multiple soft tissue abscesses (white arrows). (d, e) Axial T1-W FS gadolinium-enhanced images better delineate multiple abscesses and periosteal fluid, along with the cloaca (arrow)

Fig. 19.5
Acute osteomyelitis with intraosseous, subperiosteal, and soft tissue abscesses in a 12-year-old. Axial T1-W FS (a) and gadolinium-enhanced T1-W FS (b) images show irregular tibial marrow enhancement and extensive soft tissue enhancement, with central non-enhancement both within the marrow (black arrow) and in the soft tissues lateral to the tibia (white arrow), indicating osseous and soft tissue abscesses. (c) The axial T2-W FS image shows increased signal in portion of marrow that does not enhance, with extensive soft tissue inflammation and a subperiosteal abscess (arrow). (d) Coronal gadolinium-enhanced FS T1-W image also shows intraosseous abscesses as well as the subperiosteal abscess. (e) Four weeks later, the distal tibia appears permeative, with a well-defined thin line of periosteal reaction (arrows)
Brodie abscess, characteristic of subacute osteomyelitis, is composed of a central lucent focus in the metaphysis surrounded by sclerosis and accompanied by a tortuous channel that may drain to the physis (Figs. 19.6, 19.7, 19.8, and 19.9); identification of this channel confirms the diagnosis of subacute osteomyelitis [7]. Single or laminated periosteal new bone formation is typical, with little surrounding inflammation [6]. Epiphyseal subacute osteomyelitis usually appears as a lytic, cystic, intra-epiphyseal lesion.
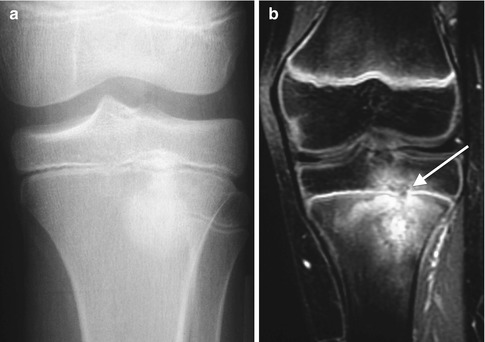
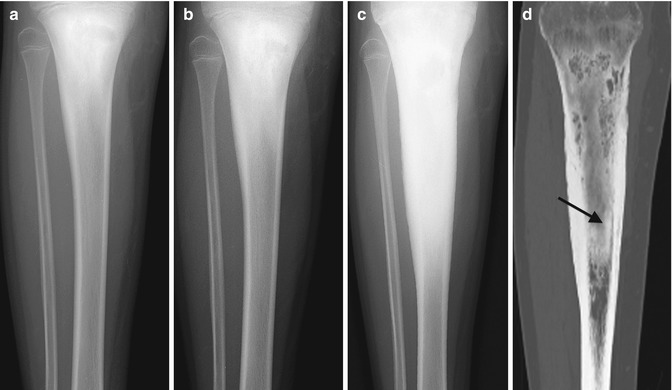
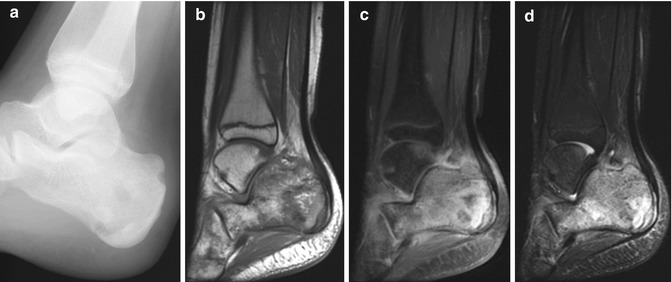
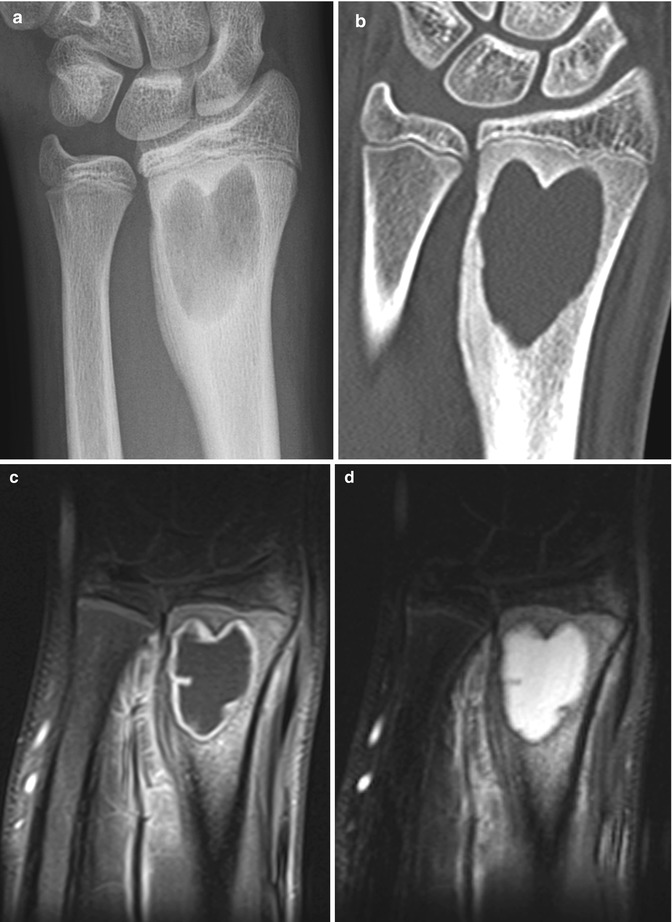

Fig. 19.6
Subacute osteomyelitis in a teenage boy. (a) Osteolysis surrounds sclerosis in this less classic case. (b) Coronal gadolinium-enhanced T1-W FS MRI shows layered enhancement, with a cloaca (arrow) breaking across the physis along with epiphyseal involvement. Note the relative lack of soft tissue enhancement

Fig. 19.7
Evolution of Staphylococcus aureus osteomyelitis. (a) Radiograph of subacute stage shows ill-defined decreased density with surrounding sclerosis in the proximal tibial metaphysis. (b) Several months later, there is diffuse sclerosis at the mid and proximal tibia with central lucency consistent with subacute to chronic osteomyelitis. (c) Three years later, sclerosis and thickening have progressed to chronic osteomyelitis. (d) Coronal reformatted CT shows a sequestrum (arrow)

Fig. 19.8
Subacute osteomyelitis in a 12-year-old girl with 6 weeks’ history of cellulitis and pain. (a) Lucent focus is seen posteriorly within the heterogeneous calcaneus. (b) Sagittal T1-W MRI shows a layered appearance to the calcaneus. (c) T1-W gadolinium-enhanced FS MRI shows osseous and limited soft tissue enhancement. (d) Short-tau inversion recovery sequence shows extensive calcaneal edema, which has a geographic appearance

Fig. 19.9
Brodie abscess in a 14-year-old boy with wrist pain. (a) There is a well-circumscribed lucency in the distal radial metaphysis, with adjacent eburnated bone. (b) Coronally reformatted CT shows the abscess extending to the physis, with surrounding eburnation and maturing lamellar periosteal new bone. (c) T1-W gadolinium-enhanced FS image shows smooth marginal enhancement at the fluid collection and slight marrow and soft tissue enhancement. (d) Coronal T2-W FS MRI shows hyperintense fluid in the abscess but minimal marrow edema
Chronic osteomyelitis manifests with thick periosteum and cortex, forming the involucrum that encases the sclerotic sequestrum (Figs. 19.10 and 19.11). The prominent cortical thickening is termed “Garré sclerosing osteomyelitis.”
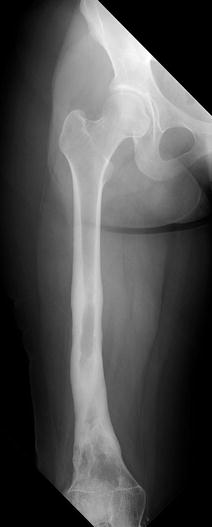
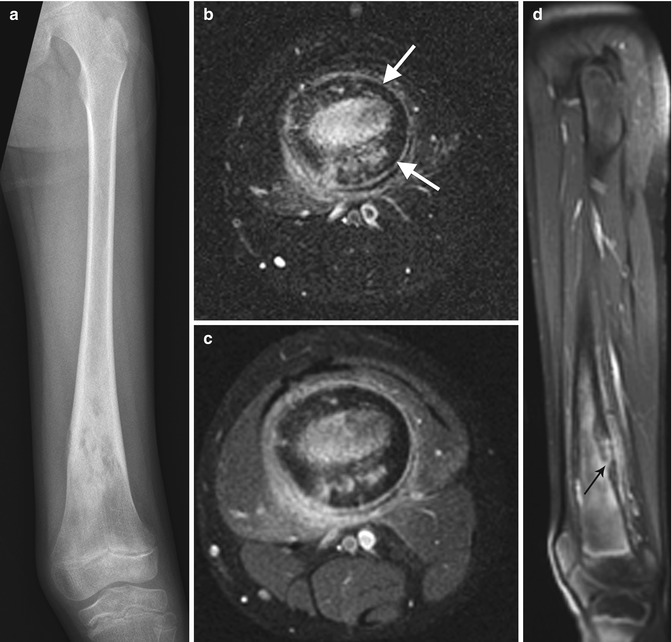

Fig. 19.10
Chronic osteomyelitis in a 15-year-old girl, refractory to treatment. There is marked endosteal sclerosis and a thick, undulating cortex

Fig. 19.11
Chronic osteomyelitis. (a) Diaphyseal and metaphyseal sclerosis in the distal femur. The thickened cortex is hypointense (arrows) on both axial T2-W fat-suppressed (b) and axial T1-W gadolinium-enhanced fat-saturated MRI (c), with relatively mild edema and enhancement in surrounding soft tissues. (d) Sagittal FS T1-W gadolinium-enhanced image shows a draining cloaca that enhances as it penetrates the posterior femoral cortex (arrow)
Scintigraphy
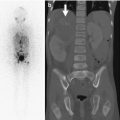
Some centers advocate scintigraphy for diagnosis of osteomyelitis, performing magnetic resonance imaging (MRI) only in cases that involve the spine or pelvis or do not respond to treatment [10]. Three-phase bone scan may be easier to obtain and less expensive than other advanced imaging, and its sensitivity exceeds 80 %, but it is only 50 % specific [11]. Able to discern as little as 5 % change in bone turnover, a bone scan can be positive 24–48 h from onset of symptoms. The bone scan is especially useful in infants and other patients who cannot localize disease—such as neonates (Fig. 19.12) in whom 22 % of disease is multifocal [12]. Furthermore, in the 10 % of patients in whom the site of disease does not correspond to the painful locus, a whole-body bone scan should identify the disease (as will a whole-body MRI) [10].
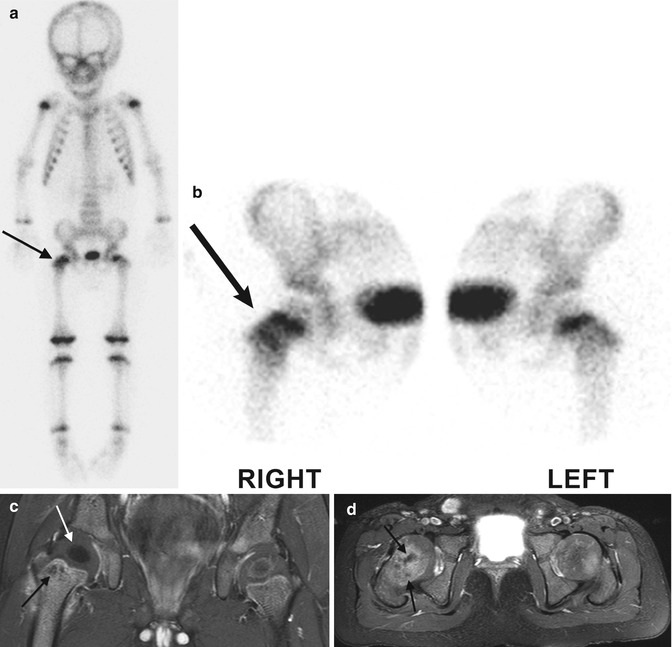

Fig. 19.12
Acute osteomyelitis in an infant. (a) Delayed whole-body Technetium-99m-labeled bone scan shows increased activity in the right hip (arrow). Pinhole views (b) localize the increased activity to the femoral neck and intertrochanteric region. Coronal (c) and axial (d) T1-W gadolinium-enhanced FS MRI show marrow and soft tissue enhancement. Non-enhancing foci in the femoral neck (black arrows) may represent abscesses, whereas the T1-hypointense right femoral head ossific nucleus (white arrow) is likely ischemic (Courtesy of Tal Laor, Cincinnati Children’s Hospital)
A triple-phase bone scan should be performed. Osteomyelitis has increased uptake in all three phases (Fig. 19.13). However, the delayed images are most definitive, and excluding unusual locations such as the pelvis (see Fig. 19.18 below) normal delayed images usually exclude the diagnosis of osteomyelitis. Pinhole images are helpful. Single photo emission computed tomography (SPECT) improves spatial resolution but can be difficult to perform in children [13].
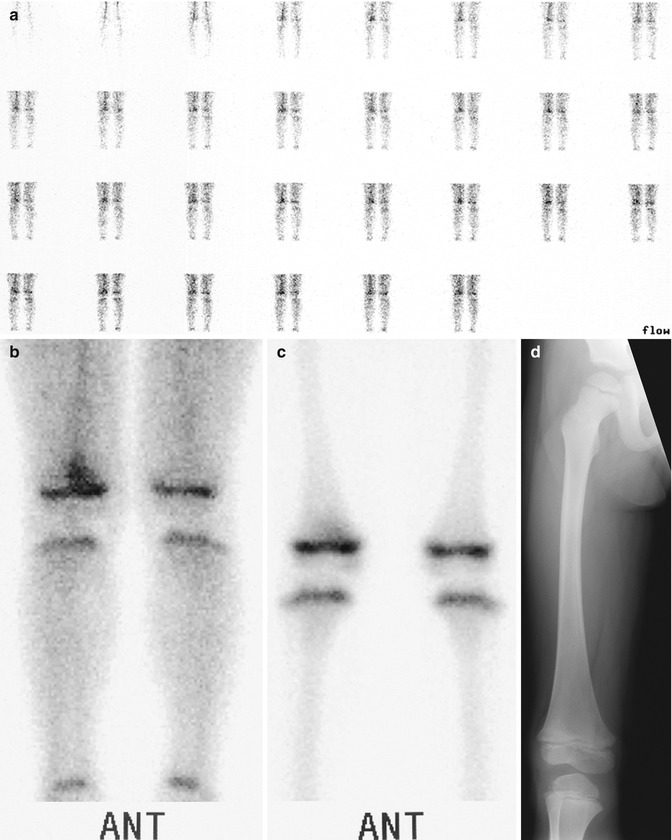

Fig. 19.13
Acute osteomyelitis in a 4-year-old boy with right knee pain after receiving a course of antibiotics for strep throat. (a) Blood flow image in a Technetium-99m-labeled three-phase bone scan shows increased flow to the right leg. (b) Blood pool shows focal increased activity at the distal right femur. (c) Delayed image shows persistent increased activity, localized to the distal femoral metaphysis. (d) The contemporaneous radiograph is normal (Courtesy of Alan H. Siegel, MD, Dartmouth Hitchcock Medical Center)
Bone scans may not show disease that abuts the physis, and ischemia or a weak inflammatory response can result in a false-negative examination. Increased pressure or marrow ischemia can lead to decreased blood flow resulting in “cold” osteomyelitis and necrosis due to vascular thrombosis; both are more common with MRSA and might not be recognized at bone scan [14]. Findings of infection, tumor, and trauma can overlap. Gallium improves specificity to 80 % [6] and may delineate soft tissue abscesses, but it is less desirable than MRI, in part due to high radiation dose.
Because scintigraphy cannot delineate abscesses, it is generally less useful in the spine and pelvis, where abscesses are especially common (again, gallium may be employed). In the spine, delineation of intraspinous abscesses and possible cord compression is essential. Abscesses are less often associated with osteomyelitis of the long bones. Scintigraphy may be sufficient in these cases, provided that if there is a poor response to antibiotic therapy, then MRI is performed [10].
Spatial resolution has improved with fludeoxyglucose photon emission tomography (FDG-PET) and SPECT, allowing better differentiation of osteomyelitis from septic arthritis and cellulitis; specificity of combined SPECT/CT exceeds 80 % [11, 15]. If complementary white blood cell scanning is performed, then sensitivity and specificity exceed 90 %, as the white blood cell (WBC) scan is positive in marrow that is cold on SPECT because the normal marrow has been replaced with an infectious process. However, the application of WBC scintigraphy to pediatric imaging is limited, as it usually requires at least 15 mL of blood [13]. PET/CT may be useful in follow-up of acute osteomyelitis, as it can differentiate between reparative activity and acute infection [16]. It may also allow identification of a focus of active infection within chronic osteomyelitis.
Ultrasound
Ultrasound can depict juxtacortical soft tissue swelling as soon as 1 or 2 days after the onset of symptoms [17], well before radiographic changes are evident. In the early stages, soft tissue inflammation appears as loss of normal tissue planes along with interdigitating areas of decreased echogenicity. If subperiosteal fluid is present, bone is presumed to be involved [18]. Lentiform subperiosteal fluid collections measuring at least 2 mm in thickness, which may be of increased or decreased echogenicity, indicate disease progression and the development of subperiosteal abscesses [18] (Fig. 19.4). With further progression, superficial cortical bone defects, fistulas, and sequestra can become apparent [17, 18]. Color Doppler shows increased blood flow as soon as 4 days after the onset of symptoms [18] and may permit monitoring of disease progression by evaluating blood flow in soft tissues around infected periosteum [19]. It is important to recognize that a negative ultrasound does not exclude osteomyelitis.
CT
MRI has generally supplanted computed tomography (CT) for evaluation of acute osteomyelitis, but in cases of chronic osteomyelitis, CT is helpful, as it readily depicts foci of intraosseous gas, cortical thickening, sclerosis, encroachment on the medullary cavity, and draining sinus (Figs. 19.7 and 19.14). It also identifies not only large sequestra but also the smaller sequestra and foreign bodies that may not be appreciated at MRI but that must also be removed in order for antibiotic therapy to succeed [7].
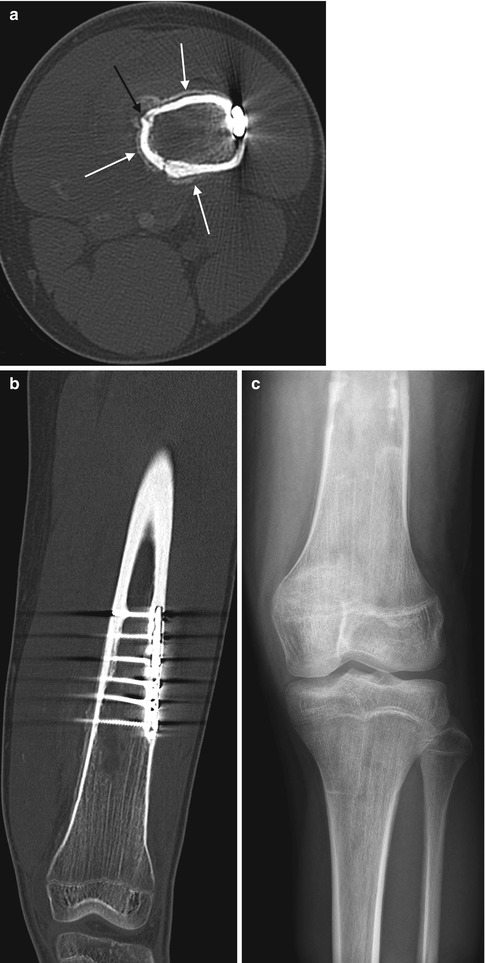

Fig. 19.14
Chronic osteomyelitis in a 15-year-old boy with knee pain 6 years after plate and screw fixation of a femoral fracture. (a) Axial CT shows periosteal new bone (white arrows) with a cloaca (black arrow). (b) Coronal reformatted CT reveals the extent of the abnormality. (c) One month later, hardware has been removed, and there is patchy lysis and sclerosis of the distal femoral diametaphysis
MRI
MRI is widely used to assess musculoskeletal infection, as its inherent tissue contrast, multiplanar imaging, and ability to differentiate soft tissue from bone infection lead to high sensitivity and specificity. After screening radiographs, it is often the next diagnostic study. If symptoms of acute osteomyelitis persist for more than 48 h after initiation of antibiotic treatment, MRI is advisable to determine response to therapy, to evaluate for abscess formation, and to assist with surgical planning [20]. Locations with complex anatomy, such as the spine and pelvis, also benefit from early MRI.
The protocol includes T1, T2 with fat suppression, and short-tau inversion recovery (STIR) in orthogonal planes. Obtaining both pre- and postcontrast T1-weighted (T1-W) images facilitates correct interpretation of findings, but administration of gadolinium is somewhat controversial. Diffusion-weighted imaging is useful, increasing conspicuity.
Marrow signal alters 1–2 days after onset of symptoms. However, signal changes are more subtle in children than in adults due to the higher water content in their bone marrow, and fluid-sensitive sequences can be misleading as both normal and abnormal marrow can be hyperintense. T1-W sequences better delineate marrow morphology [12], and a wide zone of transition is usually apparent. Edema, inflammatory cells, and exudate appear as ill-defined low T1 signal and as high-intensity signal on fluid-sensitive sequences (T2 and STIR) (Figs. 19.3, 19.4, 19.5, and 19.6). MRI may overestimate the extent of bony involvement, as reactive inflammation has a similar appearance [4]. Cortical bone may lose its normal low signal, and high T2 signal may be seen around the cortex, due to layering periosteal fluid. Epiphyseal and physeal involvement is readily seen on sagittal and coronal scans. If subperiosteal abscesses form, high T2/low T1 lentiform fluid collections are evident. Cortical thickening is not present in acute infection [7].
There has been considerable debate about whether gadolinium should be administered to assess osteomyelitis; many authors conclude that gadolinium is unnecessary if T1- and T2-weighted (T2-W) sequences are normal. However, if edema is present, the addition of contrast helps delineate epiphyseal involvement and also the presence of intraosseous, subperiosteal, and soft tissue abscesses. This both improves diagnostic confidence and affects management, as the presence of abscesses mandates surgical drainage [21, 22]. Devascularized bone due to septic thrombosis of nutrient channels also fails to enhance but does not demonstrate the typical rim enhancement of an abscess. Distribution of antibiotics mirrors that of gadolinium, with important implications for treatment [21]. MRI is especially useful in Staphylococcus aureus osteomyelitis, which tends to be more severe and is accompanied with a higher incidence of soft tissue complications [14].
Fat globules, larger than lipocytes, are seen in 70 % of cases of acute osteomyelitis, perhaps due to coalescence of lipid from lipocytes that have undergone necrosis. However, this is not pathognomonic for osteomyelitis, as fat globules can also be seen in sarcomas after treatment, in infarcts, and in Gaucher disease [23]. An extraosseous fat-fluid level may be pathognomonic for osteomyelitis [24]. Extraosseous fat is more common during and after puberty [25].
Although follow-up MRI is generally not necessary, it is useful in patients whose C-reactive protein does not fall within 14 days or in those with an otherwise worsening clinical course who may require repeat surgical drainage [26].
Differential Diagnosis
Aside from joint pathology such as soft tissue infection and arthritis, differential considerations for acute osteomyelitis include osteoid osteoma, osteosarcoma, Ewing sarcoma, leukemia/lymphoma, metastatic neuroblastoma, and Langerhans cell histiocytosis. The wide zone of transition on T1-W imaging helps differentiate osteomyelitis from these entities. It is also characterized by a more heterogeneous pattern of enhancement.
Subacute osteomyelitis bears some resemblance to tumor, having a more geographic appearance than does acute osteomyelitis at T1-W imaging. Several MRI characteristics help differentiate subacute osteomyelitis: the presence of multiple distinct layers and the so-called penumbra, where a central low T1/high T2 signal abscess cavity is surrounded by a two-layered rim (Figs. 19.6 and 19.8). The inner rim of granulation tissue is isointense to muscle on T1-W imaging and hyperintense on T2, whereas the outer fibrous rim is hypointense on all sequences. A halo of T1-hypointense edema surrounds the hypointense rim [27]. The penumbra consists of a thin layer of granulation tissue that surrounds the abscess. On unenhanced T1-W sequences, it is slightly hyperintense, perhaps due to its relatively low water content compared to the more central abscess or the surrounding edema; it enhances intensely [28]. The penumbra is 75 % sensitive and specific for subacute osteomyelitis and can be useful for differentiation from tumor. Fat globules are not encountered in subacute osteomyelitis [23, 29].
Differential considerations include osteoid osteoma, stress fracture, and—less likely—Ewing sarcoma, lymphoma, or metastatic neuroblastoma. Epiphyseal subacute osteomyelitis may be mistaken for chondroblastoma.
Bone sclerosis and cortical thickening in chronic osteomyelitis are hypointense on all sequences. The sequestrum and involucrum do not enhance but are surrounded by enhancing granulation tissue (Fig. 19.11). Sinus tracts are hypointense on T1 and hyperintense on T2, and their margins demonstrate enhancement. Marrow edema is present, with a sharp, well-defined margin between normal and abnormal marrow (in acute osteomyelitis, the zone of transition is ill-defined) [7]. Fat signal—if present—is due to the restoration of normal marrow [23]. Primary differential considerations are osteosarcoma and Ewing sarcoma.
Long-term sequelae include restricted or angular growth secondary to physeal damage and resultant early growth plate fusion. Alternatively, limb overgrowth can occur, as a result of hyperemia. Epiphyseal avascular necrosis or destruction decreases growth and produces severe deformity (Fig. 19.15). MRI or CT can be useful to analyze physeal damage as well as to assess the condition of the epiphysis. Long-term follow-up radiographs assess the impact on growth and angular deformity (Fig. 19.16).
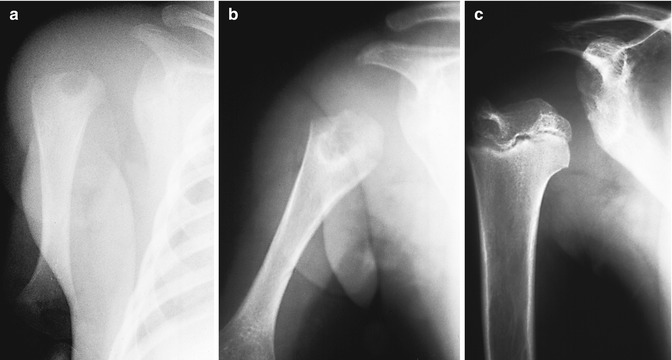
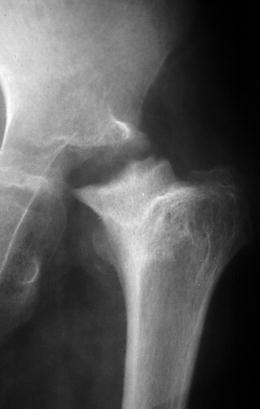

Fig. 19.15
Osteomyelitis in a premature infant. (a) There is lucency in the proximal humeral metaphysis. Although the epiphysis is not yet ossified, the humerus is displaced laterally by a joint effusion, suggesting epiphyseal involvement as well. (b) One month later, the lesion has enlarged, and surrounding sclerosis indicates reactive bone formation. Lateral displacement has resolved, and interval growth results in apparent distal migration of the lucent focus. (c) Several years later, the humeral head is small, deformed, and displaced laterally

Fig. 19.16
Late sequelae of neonatal osteomyelitis of the hip. The femoral epiphysis has been destroyed, and there is very little space between the deformed acetabulum and the sclerotic, deformed metaphysis
2 Primary Epiphyseal Osteomyelitis
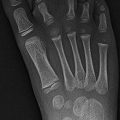
Involvement of the epiphyseal ossification center is common in infants, secondary to extension from the metaphysis. There is also an uncommon subacute primary form that occurs in early childhood; this follows direct hematogenous seeding of the epiphysis [30]. Pain and limp are often recurrent. The knee is affected almost exclusively, with distal femoral epiphyseal involvement more likely than proximal tibia. Staphylococcus aureus is the most common pathogen.
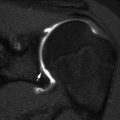
A well-circumscribed epiphyseal lucency is seen (Fig. 19.17), usually without any other bone involvement, and nuclear scintigraphy is positive. MRI demonstrates typical findings of osteomyelitis [30]. Differential diagnoses are limited and include chondroblastoma, epiphyseal osteoid osteoma, and normal developmental epiphyseal lucencies [31].
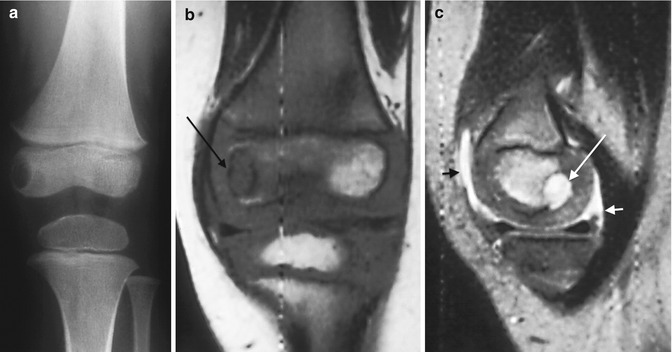

Fig. 19.17
Primary epiphyseal osteomyelitis. (a) Well-circumscribed lucency in the medial femoral condyle. (b) T1-W MRI shows hypointensity in the epiphysis, most pronounced in the medial condyle (arrow), sparing a portion of the lateral condyle. (c) Sagittal T2-W image shows fluid signal within the lesion (long arrow), which spans the ossified and cartilaginous portions of the epiphysis. Small joint effusion (short arrows)
3 Pelvic Osteomyelitis
Pelvic osteomyelitis accounts for 10 % of cases of acute osteomyelitis and tends to occur in older males (average age 7–14 years) [32]. There is often a history of trauma [33]. Signs and symptoms vary, and as many as 50 % are afebrile; diagnosis is often delayed, with symptoms initially attributed to septic arthritis, slipped capital femoral epiphysis, Legg-Perthes disease, appendicitis, or tubo-ovarian abscess [32, 34]. Abscesses are especially common in pelvic osteomyelitis, perhaps because of the typical delay (average 12 days) in diagnosis [35]. An ESR of more than 55 may help predict the presence of an abscess [32].
Osteomyelitis in the pelvis occurs in metaphyseal equivalents that border cartilage, where—as in the metaphyses of long bones—sluggish blood flow in venous sinusoids favors bacterial deposition and proliferation. Prior to skeletal maturation, the bony margins of the triradiate cartilage and ischiopubic synchondroses are common sites of pelvic osteomyelitis, followed by the sacroiliac joint, pubic symphysis, ischium, and iliac apophysis [32–34].
Imaging
Radiographs are usually negative, and ultrasound rarely succeeds in assessing deep osseous and soft tissue structures of the pelvis, although it may demonstrate a hip effusion in the setting of acetabular osteomyelitis. Scintigraphy is sensitive for osteomyelitis but fails to delineate abscesses and may underestimate disease (Fig. 19.18). It is therefore of especially limited utility in the pelvis, where abscesses are so common [10]. CT is more sensitive than radiography, but early findings are nonspecific. As infection progresses, intramedullary density increases due to reactive thickening of trabeculae [34].
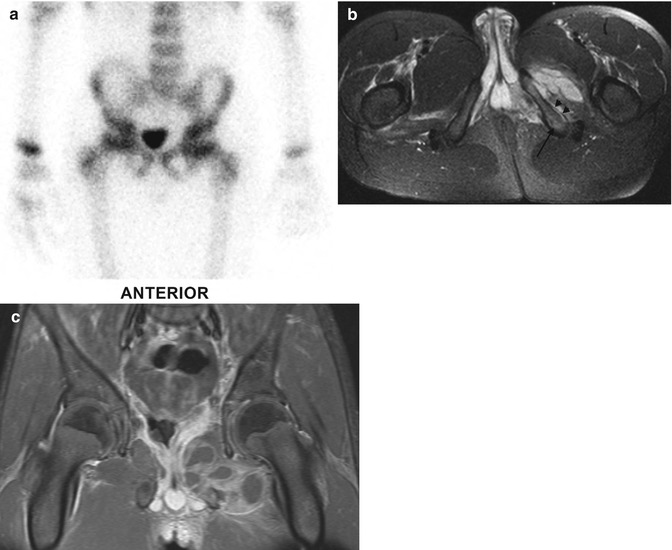

Fig. 19.18




Ischiopubic osteomyelitis with soft tissue abscesses, underestimated by bone scan. (a) Bone scan is normal. (b) Axial T2-W FS MRI shows slight edema of the left inferior ischiopubic ramus, (arrow) subperiosteal fluid (short arrows), and increased signal with fluid collections in the internal and external obturator muscles. (c) Coronal T1-W gadolinium-enhanced FS MRI shows multiple rim-enhancing intramuscular abscesses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree