Clinical and Pathologic Features
Pyogenic (septic) arthritis is usually (80% to 90%) monoarticular with predilection for large weight-bearing joints, such as the knee or hip. The clinical signs and symptoms depend on the site and extent of involvement as well as the specific infectious organism. Although most cases of septic arthritis are caused by Staphylococcus aureus, Escherichia coli, and Neisseria gonorrhoeae, other pathogens—including Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae, Candida albicans, and Serratia marcescens—are being encountered with increasing frequency in joint infections in drug users caused by the contamination of injected drugs or needles. New strains of Staphylococcus aureus may be methicillin (MRSA) or vancomycin resistant. MRSA’s secretion of the necrotizing toxin Panton-Valentine leukocidin results in more invasive infection and higher complication rates. Any large or small joint can be affected by septic arthritis, and hematogenous spread in drug addicts is characterized by unusual locations of the lesion, such as the spine (vertebrae and intervertebral disks), sacroiliac joints, sternoclavicular and acromioclavicular articulations, and pubic symphysis. Furthermore, with the increased usage of biologics and immunosuppressive drugs, clinicians should always keep in mind the possibility that an autoimmune disease has become complicated by an infection. This statement is particularly valid for the patients with rheumatoid arthritis who underwent surgery.
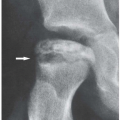
The classic clinical presentation of septic arthritis is an abrupt, acute onset of joint pain accompanied by swelling and warm sensation. The joint becomes tender to palpation, and there is marked restriction of both active and passive motion. Fever is encountered in about 60% to 80% of cases. Some patients may experience chills. Laboratory findings include elevation of white blood cell (WBC) count, elevation of erythrocyte sedimentation rate, and positive blood cultures. Pathologic findings include progressive and increasing neutrophilic infiltrate of the synovium, associated with vascular dilatation and congestion, and increased number of type A and B synoviocytes. This is followed by cartilage necrosis and subchondral bone erosion. Infiltrates of polymorphonuclear leukocytes on the articular cartilage surface are a common finding (
Fig. 9.3). Synovial fluid appears opaque or frankly purulent. The nucleated cell count is very high, up to 50,000 cells per mm
3 (see also discussion in
Chapter 1). Percutaneous aspiration and US-guided, CT-guided, or fluoroscopy-guided biopsy of a suspected focus of infection may be performed in the radiology suite. It can rapidly confirm a suspected diagnosis of infection and reveal the causative organism. For bacteriologic examination, synovial fluid should be placed into an empty sterile container (not carrying any media) and taken immediately to the laboratory for gram staining
and plating on appropriate media. Chocolate agar (CHOC), Thayer-Martin, or Transgrow media should be used when gonococcal arthritis is considered; Löwenstein-Jensen media, when tuberculous arthritis is suspected; Mannitol salt agar, for staphylococcus infection; and potato dextrose agar or Sabouraud agar for fungi infection.
Imaging Features
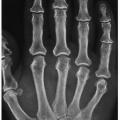
In most instances, conventional radiography is sufficient to demonstrate the pertinent features of a bone or joint infection, particularly with the advantage of digital radiography and newest technology of PACS (Picture Archive and Communication System) allowing filmless high-resolution image-display format. Most infectious arthritides demonstrate a very similar radiographic picture, including joint effusion and destruction of cartilage and subchondral bone with consequent joint space narrowing (
Fig. 9.4, see also
Fig. 3.19). However, certain radiographic features are characteristic of individual infectious processes as demonstrated at various target sites (
Table 9.2) and may be helpful in arriving at the correct diagnosis. Generally, a single joint is affected, most commonly a weight-bearing joint like the knee or hip. The early stage of joint infection may be seen simply as joint effusion, soft tissue swelling, and periarticular osteoporosis, but “radiographic” joint space is usually preserved (
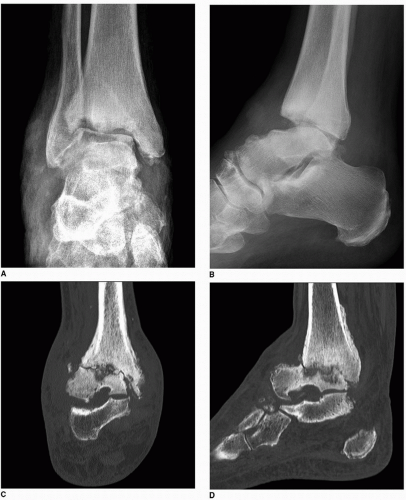
Fig. 9.5). In the later phase of pyogenic arthritis, articular cartilage is destroyed; characteristically, both subarticular plates are involved and the joint space narrows (
Figs. 9.6,
9.7,
9.8,
9.9).
Scintigraphy has a very prominent role in diagnosing musculoskeletal infections, including infectious arthritis. In cases of suspected infection, radionuclide bone scan using
technetium-99m-labeled (
99mTc) phosphonates is routinely used, because there is an accumulation of tracer in the infected areas (see
Fig. 2.54). A three- or four-phase technique is particularly useful for distinguishing infected joint from infected periarticular soft tissues if radiography is not diagnostic. With cellulitis, diffuse increased uptake is present in the first two phases, but there is no significant increase in uptake in the bone in the third and fourth delayed phases (see
Fig. 2.53). Conversely, osteomyelitis causes focally increased uptake in all four phases. The fourphase bone scan can also be useful in diagnosing septic arthritis in situ or with extension into the adjacent bone.
Once the bone sustains an injury, such as surgery, fracture, or neuropathic osteoarthropathy, that causes increased bone turnover, routine scintigraphy with technetium-labeled phosphonate becomes less specific for infection. However, radionuclide studies using gallium (a ferric analog) and indium are more specific in these instances. There is still no general agreement on the exact mechanism of gallium localization in infected tissues. After intravenous injections of gallium, more than 99% is bound to various plasma proteins, including transferrin, haptoglobin, lactoferrin, albumin, and ferritin. At least five mechanisms of gallium transfer from the plasma into inflammatory exudates and cells have been suggested. These include direct leukocyte uptake, direct bacteria uptake, the protein-bound tissue uptake, increased vascularity, and increased bone turnover. Because gallium binds to the iron-binding molecule transferrin, the mechanism of gallium uptake in infectious processes is best explained by hyperemia and elevated permeability that increase delivery of
the protein-bound tracer transferrin into the area of inflammation. Cells associated with the inflammatory response, particularly polymorphonuclear white cells in which lactoferrin is carried within intracytoplasmic granules, deposit iron-binding proteins extracellularly at the site of inflammation, serving to combat the infection by sequestering needed iron from bacteria. Lactoferrin, which has a high binding affinity for iron, takes the gallium away from the transferrin.
The other tracer used in infections is indium. Because indium-labeled white blood cells are usually not incorporated into areas of increased bone turnover, scintigraphy with indium-111 (
111In) oxine-labeled leukocytes is used as a sensitive and specific test in the general diagnosis of infection of the musculoskeletal system and in specific instances when infection complicates previous fracture or surgery. Like other imaging procedures in nuclear medicine, this test monitors the internal distribution of a tracer agent to provide diagnostic information. The inherent ability of white blood cells to accumulate at sites of inflammation makes their use in this test particularly effective in the diagnosis of infections (
Fig. 9.10, see also
Fig. 2.62). Merkel reported the sensitivity of indium scintigraphy in detecting infections to be 83%,
with a specificity of 94% and an accuracy of 88%. It must be stressed, however, that because the
111In-labeled leukocytes also accumulate in active bone marrow, the sensitivity for the detection of chronic osteomyelitis is reduced. To improve the diagnostic ability of this technique, a combined
99mTc-sulfur colloid bone marrow/
111In-labeled leukocyte study is advocated. A particularly difficult problem is the patient with diabetic foot neuropathy in whom superimposed infection is suspected. In this circumstance, radiography and even MRI are not very specific. Although soft tissue infection can be detected by the latter technique, early changes of osteomyelitis and septic arthritis may be missed. Often, no single imaging method can provide the correct diagnosis, and a combination of several imaging techniques should be used. The traditional sequential use of
67Ga citrate in conjunction with the
99mTc-methylene diphosphonate (MDP) bone scan as an aid to diagnose osteomyelitis and infectious arthritis in the diabetic foot has been supplanted in recent years by the use of
111In-labeled leukocytes. The drawback of this technique is that there remain difficulties in differentiating infection in the bone (osteomyelitis) from that in the adjacent tissue (cellulitis). A more recent attempt to improve this situation is the use of a combined
99mTc-bone scan/
111In-labeled leukocyte study to determine whether the leukocyte collection is in the bone or in the soft tissue. A new challenger to
111In leukocyte scanning is the
99mTc-hexamethylpropylene amino oxine (HMPAO)-labeled leukocyte scan. At the time of this writing, other methods are being tested, namely, isotope-labeled (
99mTc,
111In, or
123I) monoclonal antigranulocyte antibodies, isotope-labeled polyclonal IgG, isotope-labeled monocytes, isotope-labeled chemotactic polypeptide analogs, and isotope-labeled specific antibodies against bacteria.
Over the past decade, FDG-PET/CT has emerged as a rapidly evolving diagnostic tool for evaluation of infections. In particular, recent studies by Diaz and collaborators using a combination of [124I] FIAU-positron emission tomography and CT demonstrated positive signals at the site of infection at two hours after administration of 2mCi (74 MBq) of radiopharmaceutical agent. FIAU [1-(2′-deoxy-2″fluoro-beta-D-arabinofuranosyl)-5-iodouracil], a nucleoside analog that freely enters and exits cells, is a substrate for the native thymidine kinase (TK) from a wide variety of bacteria. Once phosphorylated by TK, [124 I] FIAU becomes trapped within bacteria and can be detected with PET/CT.
Arthrography has rather limited application in the diagnosis of joint infections. This radiologic technique, which is often performed after aspiration of the joint to obtain a fluid specimen for bacteriologic examination, helps determine the extent of joint destruction and demonstrate the presence of synovitis (see
Fig. 2.30). CT plays a determining role in demonstrating the extent of infection in bones and joints (
Figs. 9.11 and
9.12). Ultrasound (US) can occasionally be used in diagnosing soft tissue and joint infections, as well as osteomyelitis. This modality has the advantage of being easily accessible and available at relatively reasonable cost. In addition, this technique does not expose the patient to ionizing radiation. Real-time capability of US is unique in providing a means to evaluate structures under dynamic conditions. Furthermore, US plays an important role in the guidance of percutaneous biopsy and aspiration of infectious lesions, as well as the therapeutic drainage of abscesses.
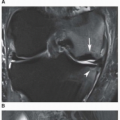
At the present time, MRI established its place in the evaluation of musculoskeletal infections. As several studies have indicated, osteomyelitis, soft tissue abscesses, joint and tendon sheath effusions, and various forms of cellulitis are well depicted by this modality. MRI is as sensitive as
99mTc-MDP in demonstrating osteomyelitis and infectious arthritis and more sensitive and more specific than other scintigraphic
techniques in demonstrating soft tissue infections, primarily because of its superior spatial resolution. The proper evaluation of musculoskeletal infections with MRI requires both T1- and T2-weighted images in at least two imaging planes. In anatomically complex areas such as the pelvis, spine, foot, and hand, three planes may be necessary. MRI manifestations of pyogenic arthritis include joint effusion with surrounding soft tissue edema and bone marrow edema (
Figs. 9.13 and
9.14). In more advanced stages, cartilage and bone destruction may be seen, due to associated osteomyelitis (
Figs. 9.15,
9.16,
9.17, see also
Fig. 9.4). “Lamellated” joint effusion demonstrated with MRI has been described as a reliable sign of septic arthritis.