Myocarditis (from Greek/Latin “inflammation of the heart muscle”) is commonly used for viral myocarditis, although there are numerous other heart conditions leading to myocardial inflammation, including acute ischemic injury, infiltrative diseases, allergies, and toxic or mechanical injuries. Thus inflammatory tissue pathology is not specific to a viral etiology and any such tissue pathology visualized by cardiovascular magnetic resonance (CMR) by itself is not specific as well. Myocardial inflammation is accompanied by hyperemia and leukocyte infiltration, as well as edema and necrosis, as typical features of reversible or irreversible cellular injury.
Patients with myocarditis may be asymptomatic or oligosymptomatic, although the frequency of electrocardiogram (ECG) changes in systemic viral illness indicates a high rate of myocardial involvement in systemic viral disease. The clinical presentation of myocardial inflammation may vary from subtle illness to overt heart failure or sudden death. Symptomatic patients may present with chest pain (caused by inflammatory neural stimulation itself), dyspnea, fatigue (both potentially reflecting ventricular dysfunction with an absolute or relative decrease of cardiac output), palpitations (extrasystole), or malaise.
In routine clinical practice, transthoracic echocardiography (TTE) is frequently used to assess left ventricular (LV) parameters. It is widely available, noninvasive, fast, and straightforward to perform in most patients. Its quantitative and diagnostic accuracy, however, suffers from a frequently poor ultrasound transmission of lungs and bones, angular errors of the imaging plane, difficulties in placement of the imaging plane, problems in identifying the endocardial border, and parallel shift of the ultrasonic plane out of the targeted center. TTE does not provide relevant information on the myocardial tissue.
Cardiovascular Magnetic Resonance Approach to the Patient With Myocarditis
CMR is regarded as the most useful noninvasive clinical tool to assess myocarditis, and its diagnostic accuracy may exceed that of other available invasive and noninvasive approaches. Therefore using proven protocols, it should be a first-line diagnostic tool in centers with access to CMR. This is because of the capabilities of CMR, which is the gold standard for noninvasively quantifying biventricular mass, volume, and function, and is especially useful for assessing tissue pathology in vivo. As in other forms of cardiomyopathies, the spatial distribution of tissue abnormalities is of special importance and, consequently, the diagnosis of myocardial inflammation in CMR is mostly based on visualizing hyperemia, edema, necrosis or scar, and, as additional supporting features, pericardial effusion and ventricular dysfunction. If properly used, CMR can identify or rule out myocardial inflammation, assess its acuity and extent/severity, and even give prognostic information.
For viral myocarditis, it is important to realize that CMR does not allow for verifying or excluding viral persistence or autoimmune processes because the inflammatory response on a tissue level is very similar. In patients with severe disease and CMR evidence for severe injury, an endomyocardial biopsy may reveal giant-cell myocarditis, and immunosuppressive therapy may be advised.
Morphology and Function
In cases of uncomplicated myocarditis, LV and right ventricular (RV) function may be entirely normal, even in the presence of myocardial tissue involvement/injury. In other cases, ventricular wall motion abnormalities may be very subtle and often in the inferolateral region, which is notoriously difficult to assess by TTE. Because CMR allows for nonrestricted views and because of its very accurate quantitative assessment of related parameters, it is better suited for understanding functional abnormalities. Moreover, accurate and reproducible RV volumes and ejection quantification can be obtained. Today, balanced steady-state free precession (bSSFP) techniques offer the best contrast between blood and endocardium and thus are recommended.
Under routine clinical circumstances, a biplanar approach (two- and four-chamber view) or multiplanar approach often are sufficient, but most published data refer to the use of short-axis stacks, covering the entire left and right ventricle from the mitral plane to the apex. The slice thickness should be ≤10 mm; in case of circumscribed or subtle global changes, it should be reduced adequately. Although including trabecular tissue may improve reproducibility, results for LV volumes and mass are less accurate, especially in patients with LV hypertrophy.
Tissue Characterization
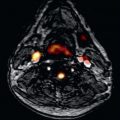
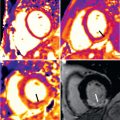
Several contrast-enhanced (CE) and noncontrast CMR approaches are available to assess the myocardial tissue. Currently, the most frequently used techniques are imaging edema and necrosis (see Figs. 35.1 and 35.2 ).
Edema: T2-Weighted Cardiovascular Magnetic Resonance
Noncontrast T2-weighted imaging using short inversion time (TI) inversion recovery techniques is a robust technique to visualize fluid accumulation such as edema and effusion in inflammatory diseases because of its specific properties. Patients with myocarditis typically show regional and/or global high signal intensity, which usually equals or exceeds that of skeletal muscle as an internal reference by a factor of 2 and is reported as the signal intensity (SI) ratio. An increased SI ratio in acute myocarditis is a good predictor of functional recovery. T2-weighted imaging of the heart is challenged by an inherently low signal-to-noise ratio (SNR) and a susceptibility to motion and arrhythmia-related artifacts. It is therefore important to (1) use thicker slices than usual (12–15, or even 20 mm) to increase SNR; (2) use a triple-inversion or double-inversion sequence and ensure an effective repetition time (TR) of at least 2000 ms to maximize T2 sensitivity; and (3) avoid the use of surface coils to prevent overcalling in anterior segments and missing disease in inferolateral segments.
Hyperemia: Early Gadolinium Enhancement
Free breathing, black-blood, fast spin echo images acquired early (for 2 minutes between 20 seconds and 3 minutes after injection) after gadolinium (Gd) injection can be used to visualize myocardial inflammation. The increased uptake is most likely caused by a combination of increased inflow (inflammatory hyperemia), slow interstitial washin/washout kinetics (capillary leakage and edema), diffusion into cells through leaky membranes (necrosis), and an increased volume of distribution (scar tissue). Long-term follow-up revealed persistence of these changes in patients with clinical and functional evidence for ongoing inflammation. An increased Gd accumulation has also been described in patients with myocardial inflammation due to Chagas myocarditis, sarcoidosis, and after doxorubicin chemotherapy. In suspected chronic myocarditis, early enhancement and edema ratio were found to be the most sensitive CMR markers for immunohistologically defined inflammatory activity.
Necrosis and Scar: Late Gadolinium Enhancement
Late gadolinium enhancement (LGE) imaging is performed using an inversion-recovery gradient echo sequence with inversion times which “null” the signal of normal myocardial tissue, with images being obtained ≥10 minutes after injection. Bright areas indicate irreversible myocardial injury, which can be acute (necrosis) or chronic (scar). Following the success in visualizing myocardial scarring in coronary artery disease, LGE has also been used to assess myocarditis with a very good agreement between CMR findings and histopathology. Importantly, the regional distribution of myocarditis lesions differs starkly from that of ischemic hyperenhancement, because—as opposed to myocardial infarcts—they generally do not involve subendocardial layers. It should be kept in mind that the sensitivity of LGE to detect myocarditis is generally lower than that of T1/early enhancement and T2-weighted CMR, whereas its regional distribution is very specific for nonischemic injury. Interestingly, necrosis/scars in myocarditis often affect lateral regions as a row of focal injuries ( Fig. 35.1 ), sometimes, however, with transmural extent ( Fig. 35.2 ).


Phase-sensitive inversion recovery (PSIR) LGE imaging usually facilitates interpretation, as it is less sensitive to an inadequately chosen inversion time.
Novel Techniques: Mapping of Myocardial T1 and T2
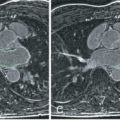
The quantification of T1 and T2 relaxation times, also referred to as parametric CMR mapping, has been proven to be a very useful option to evaluate global myocardial tissue pathology. Native (noncontrast) T1 and T2, and postcontrast T1 have been established as a biomarker of myocardial edema and fibrosis. Native T1 is modulated by numerous factors, including edema, and patients with acute myocarditis consistently exhibit an increase of T1. Fig. 35.3 shows T1 maps in comparison to edema and necrosis. Its diagnostic use in myocarditis is increasing. It appears to be able to identify affected myocardium in areas without LGE. Furthermore, it may provide confirmatory information in acute settings. Native T1 has been shown to allow differentiation between acute pheochromocytoma myocarditis, cured (operated) pheochromocytoma, and hypertensive heart disease. Interesting data have been recently provided on treated patients with HIV. A recent study provided preliminary evidence that native T1 mapping may be able to not only identify acute myocarditis but also convalescent myocarditis.