Key Points
- ▪
Cardiac CT’s ability to depict fine structural detail within the heart enables it to characterize the morphologic features of cardiomyopathies such as wall thickness, left ventricular mass, mass distribution, and chamber dimensions.
- ▪
CCT’s inability of to provide functional data beyond ejection fraction limits the functional characterization of cardiomyopathies, which would need to include characterization of atrioventricular valve insufficiency, pulmonary pressures, and outflow obstruction.
- ▪
CCT can contribute to the evaluation of undifferentiated dilated cardiomyopathy by identification or exclusion of underlying coronary artery disease.
- ▪
CCT, unlike CMR, is able to image hearts with pacemakers and implantable cardioverter defibrillators.
Recent technical advances in cardiac CT (CCT) have been driven largely by the requirements for accurate noninvasive coronary angiography. As a consequence of improved image quality and temporal resolution, attention has also turned to the broader application of cardiac CT assessment to other disease entities. The role of CT in the assessment of cardiomyopathy has, to date, been largely restricted to the measurement of left ventricle (LV) size and function. Other modalities such as echocardiography, cardiac MR (CMR), and nuclear techniques usually are favored for work-up of these patients, with additional information often obtainable with regard to concurrent disturbances such as mitral insufficiency, pulmonary hypertension, and left ventricular outflow tract obstruction. However, disease-specific morphologic features and the potential for CT-derived tissue characterization are increasing opportunities for the assessment of nonischemic myocardial disease by this rapidly evolving modality.
Limitations of noncoronary CCT at this stage include challenges in temporal resolution for functional assessment and the significant radiation exposure often incurred with the use of retrospective reconstruction techniques. Subsequently, comparatively little has been published on the assessment of cardiomyopathies by CCT. Dose modulation can play a role in radiation reduction. For morphology and tissue characterization purposes, prospective ECG-gating, multisegment acquisition/reconstruction, and wide-range detector systems allow dynamic volume assessment with significantly reduced radiation exposures. Furthermore, the development of dual-source/spectral energy imaging holds significant potential for the refinement of tissue characterization techniques with CT.
Dilated Cardiomyopathy
There is no established routine role yet for cardiac CT in the evaluation of dilated cardiomyopathies, and little role in the assessment of LV function in general, given the other means to establish it.
A potential role may arise for the negative predictive value of CCT in regard to the presence of coronary disease in cases of probable idiopathic dilated cardiomyopathy. Validation has begun in selected patients. In a single-center study, among 61 patients with dilated cardiomyopathy (DCM) and 139 patients undergoing coronary angiography, 16-slice CT yielded 99.8% negative predictive value for significant (>50%) coronary stenoses. Feasibility was 97%. The most common artifact observed in this series was a hypertrophied coronary venous system in proximity to the coronary arteries. Given the increased size of hearts with DCM and the greater spatial dispersion of the coronary tree, as well as frequent coexistence of atrial fibrillation, wide-detector CCT with the potential for single cardiac cycle acquisition may have an advantage over 64-slice CT.
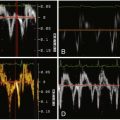
A further application may be the use of CCT before consideration of cardiac resynchronization therapy (CRT) as a single-modality assessment of cardiac function, coronary venous anatomy, regional wall motion, and scar. The presence of myocardial dyssynchrony has been assessed with CCT and shown to be reproducible and to correlate with two- and three-dimensional echocardiography measures. However, the clinical application of these techniques still must be prospectively evaluated ( Fig. 21-1 ; ).

Hypertrophic Cardiomyopathy
Cardiac CT is able to depict the distribution of hypertrophy in hypertrophic cardiomyopathy (HCM) and quantify it, as can CMR, although with the risk of radiation exposure, contrast allergy, and nephropathy. Validated by CMR, myocardial mass in HCM appears to be at least as good a predictor of cardiovascular mortality as wall thickness greater than 30 mm. Notably, 20% of phenotype HCM cases may have normal myocardial mass. Although echocardiography still is the mainstay of assessment of HCM and hypertrophic obstructive cardiomyopathy (HOCM), especially because of its versatile Doppler capabilities, some cases of HCM are difficult to image, especially at the apex, which is relevant when evaluating possible apical variant HCM. The excellent spatial resolution of CT also may facilitate the identification of myocardial crypts, which have been suggested as an early sign of underlying cardiomyopathy in patients who carry an HCM mutation. CMR or cardiac CT may thus complement echocardiographic assessment. The potential of CCT to plan and then assess the myocardial response to percutaneous transluminal septal ablation for HOCM also has been reported. Currently, myocardial fibrosis assessment by late gadolinium CMR is well documented and may play a role in risk stratification in the future. While its validation is currently limited to case reports, the potential for characterization of myocardial fibrosis by delayed contrast CT may demonstrate a similar utility.
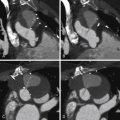
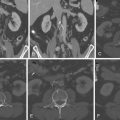
Coronary CT angiography (CTA) in persons with HCM is likely to be relatively straightforward, because many are taking β-blockers to control their HCM. Feasibility of chest pain evaluation in HCM has been suggested ( Figs. 21-2 through 21-10 ;









Infiltrative Cardiomyopathy And Tissue Characterization
Tissue characterization previously has been possible only with CMR sequences to demonstrate the following:
- □
Scar (late enhancement)
- □
Inflammation (early relative enhancement)
- □
Edema (increased T2 signal)
- □
Intramyocardial fat (suppressible T1 signal)
- □
Iron overload (short T2∗ time).
Cardiac CT late enhancement (5 to 10 minutes after contrast injection) techniques are beginning to evolve.
Using a definition of intramyocardial fat as attenuation of −30 to −190 Hounsfield units (without histologic validation), fat has been observed in both left and right normal healthy ventricles, as well as within areas of prior infarction. Dystrophic myocardial calcification diagnosed by CT in the absence of significant coronary disease also has been reported as a rare cause of congestive heart failure.
Sarcoidosis
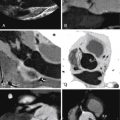
Using 64-slice CT and scanning immediately and 10 minutes after contrast injection, delayed enhancement has been shown in a case of sarcoidosis, with a nonischemic pattern similar to that seen with CMR, and colocalizing to areas of myocardial chronic sarcoidosis thinning. Fusion single-photon emission CT (SPECT)/CT may improve diagnostic accuracy compared with SPECT alone ( Figs. 21-11 through 21-13 ; ).



Amyloidosis
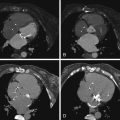
In cases of amyloidosis, delayed phase scanning (10 minutes) has been shown to yield late enhancement patterns that correspond to those seen in CMR late enhancement scanning ( Fig. 21-14 ).

Fabry DIsease
Although Fabry disease, a disorder of α-galactosidase deficiency, is most commonly assessed with cardiac MRI and echocardiography, the characteristic findings of LV hypertrophy and associated delayed contrast enhancement patterns consistent with myocardial fibrosis have also been appreciated with CCT ( Figs. 21-15 through 21-17 ).



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree