The immature brain is more prone to seize than the mature brain. Causes of seizure are multiple and affect different neuroimaging modalities. The most common associated diseases are hypoxia-ischemia, intracranial hemorrhage and cerebral infarction, central nervous system infections, and acute metabolic disturbances. Ultrasound (US) is not specific. Computed tomography (CT) carries the risk of irradiation and is not as productive as magnetic resonance (MR) imaging. MR imaging is the modality of choice; it is difficult to perform in a neonate, but it is more sensitive and versatile than US or CT, and is now widely used in specialized centers.
Seizures represent the most common clinical symptom of neurologic disorder in the neonatal period. The incidence is 1.3 to 3.5/1000 in term newborns, and as high as 10 to 130/1000 in preterm neonates. Recognizing the seizure, a well-defined epileptic syndrome, and the many possible causes is important because some symptoms may mimic normal clinical manifestations, and causes of seizure may affect the choice of neuroimaging modality. However, there are no established evidence-based guidelines for management of neonatal seizures and for the appropriate use of brain imaging. Ultrasonography (US), computed tomography (CT), and magnetic resonance (MR) imaging are widely used to image the neonatal brain, but MR (using diffusion-weighted imaging [DWI], diffusion tensor imaging [DTI], and MR spectroscopy) is likely to provide the most complete and useful information.
What to consider?
Identifying the Seizures
The immature brain is prone to seize. Neonatal seizures are defined clinically as abnormal, stereotyped, paroxysmal alterations in neurologic function occurring in the first 28 days after birth of a term neonate or before 44 weeks of gestational age in a preterm infant. This abnormal function may involve abnormal motor, sensory, or automatic activity, with or without a change in level of consciousness. Not all clinical seizures correlate with electroencephalograph (EEG) changes and not all seizures shown on EEG recordings are clinically apparent. Neonatal seizures may be epileptic or nonepileptic and are classified according to their characterization. Electroclinical findings distinguish (1) clinical seizures with consistent electrocortical signature that are considered epileptic, (2) clinical seizures without consistent electrocortical signature that are presumed nonepileptic, and (3) electrical seizures without clinical manifestation. The first group may consist of focal clonic seizures (unifocal, multifocal, hemiconvulsive, axial), focal tonic seizures (asymmetrical truncal posturing, limb posturing, sustained eye deviation), myoclonic seizures (generalized or focal), and spasms (flexor, extensor, mixed extensor and flexor). The second group includes myoclonic seizures (generalized, focal, fragmentary), generalized tonic (flexor, extensor, mixed flexor and extensor), and motor automatisms (oral-buccal-lingual movements, ocular movements, movements of progression such as swimming and bicycling, complex purposeless movements).
It may be difficult to distinguish subtle neonatal seizures from other paroxysmal neurologic manifestations of the newborn. Apnea can express a subtle seizure. Most apneic episodes in preterm infants do not represent seizure activity but are associated with a bradycardia. In contrast, convulsive apnea is not accompanied by bradycardia but by other subtle phenomena such as eye opening, staring, and eye deviation. Jitteriness is a common benign neonatal movement disorder characterized by symmetric tremors of the extremities. It is generally induced by an external stimulus and ceases with gentle restraint or passive flexion. It occurs in infants without neurologic impairment and is not necessarily an abnormal feature. It is mainly encountered in hypoxic-ischemic encephalopathy (HIE), hypoglycemia, hypocalcemia, and drug withdrawal.
Epileptic Syndromes Occurring During the Neonatal Period
Idiopathic, symptomatic or cryptogenic, neonatal seizures often are part of specific epileptic syndromes. Idiopathic epilepsies include benign familial neonatal convulsions, benign neonatal convulsions, and benign familial neonatal-infantile seizures.
Benign familial neonatal convulsions are a potassium voltage-gated channelopathy with an autosomal dominant inheritance and incomplete penetrance. This rare familial syndrome is characterized by unilateral or bilateral clonic, tonic-clonic, and apneic or tonic seizures on the second or third day of life, with normal interictal EEG. The outcome is generally favorable with seizures stopping early in life and normal psychomotor development. Some patients may develop febrile seizures or epilepsy later in life. It is genetically related to mutations of potassium channel genes KCNG2 and KCNQ3 on chromosomes 20q and 8q respectively.
Benign idiopathic neonatal seizures (also described as fifth day fits) differ from the familial syndrome: seizures occur later (fifth day of life), tonic seizures are never observed, and the interictal EEG commonly shows the pointu alternant.
Benign familial neonatal-infantile seizures are characterized by tonic or clonic seizures that occur between 2 days and 3 to 5 months, later than benign familial neonatal convulsions but earlier than benign familial infantile convulsions. This epileptic syndrome has been related to chromosomes 19q, 16p, and 2q in which mutations of SCN2A (sodium channel) are found. Symptomatic epileptic syndromes include early myoclonic encephalopathy (EME) and early infantile epileptic encephalopathy (EIEE; also known as Otahara syndrome), which are often associated with structural abnormalities. In both, the background EEG shows a suppression-burst pattern.
EIEE is characterized by a very early onset, often within the first 10 days of life. The main type of seizure is tonic spasms. Partial seizures and hemiconvulsions are also seen, whereas myoclonic seizures are rare. Seizures are accompanied by severe progressive encephalopathy that evolves to infantile spasms and Lennox-Gastaut syndrome. Most cases are associated with structural brain damage, especially hemimegalencephaly. Metabolic disorders are also reported.
EME is also characterized by a very early onset. Partial to massive myoclonus as well as partial motor seizures are the main ictal phenomena, followed by infantile spasms. The clinical course is severe with, in half of the cases, death in the first year of life. Anatomic brain abnormalities are uncommon. The most common cause is nonketotic hyperglycinemia. It is also described in sulfite oxidase deficiency. Mutations of the glutamate carrier are also a cause of EME. A high proportion of cryptogenic cases is described, and familial cases have also been reported.
Pyridoxine-dependent seizure is an autosomal recessive disorder with seizure onset between birth and age 3 months. The EEG abnormalities are nonspecific. The intravenous pyridoxine test is usually positive with cessation of seizures.
Causes
Most cases of neonatal seizures are symptomatic with associated causal and risk factors, and few neonatal seizures are idiopathic (2%–5%). The major categories of causal factors are (in decreasing order of frequency): HIE, acquired metabolic disorders (hypoglycemia, hypomagnesemia, hypocalcemia, hypernatremia, or hyponatremia), infections (meningitis, encephalitis, TORCH [toxoplasmosis, other, rubella, cytomegalovirus, and herpes]), acquired or developmental brain lesions (hemorrhage, infarction, malformations), inherited disorders, inborn error of metabolism (aminoacidurias, urea cycle defects, organic acidurias, mitochondrial disorders, peroxysomal disorders, metabolic substrate deficiencies), and maternal drug intoxication or neonatal drug withdrawal (eg, cocaine, heroin). The most common are hypoxia-ischemia, infections, hemorrhage and infarction, and acute metabolic disturbances. Infants prone to epilepsy of neonatal onset are most likely to have inborn errors of metabolism and cortical malformations.
In general, the more acute the neurologic insult, the sooner the onset of neonatal seizures. For example HIE, hemorrhages, severe metabolic disturbances, maternal drug abuse, and some cases of sepsis present seizures in the first 24 hours after birth, whereas stroke, cerebral venous thrombosis, drug withdrawal, and metabolic defects in energy production and use, such as urea cycle defects, present seizures between 24 and 72 hours after birth. Other inborn errors of metabolism, such as aminoacidopathies and organic acidopathies, cerebral malformations, or congenital lesions, tend to present seizures after 72 hours to 1 week after birth.
Do Neonatal Seizures Damage the Brain?
In animal models, neonatal seizures impair cognition and learning ability, alter behavior and memory, increase anxiety, and are associated with later epileptogenesis. Despite this strong evidence from experimental studies, the issue remains largely unanswered in humans. However, significant changes are shown with MR spectroscopy in term neonates with perinatal asphyxia, which increase along with the severity of clinical seizures. A recent clinical study suggests that clinical neonatal seizures and their treatment are associated with adverse long-term cognitive and neuromotor outcomes in perinatal asphyxia that are independent of the hypoxic-ischemic injury measured by MR imaging. Moreover, high levels of excitatory amino acids pervade the epileptic brain during the interictal state, making it particularly vulnerable to seizure-induced damage.
Brain Maturation
The neonatal brain is immature at birth and this affects neuroimaging. Brain maturation, especially brain myelination, begins before birth and continues until adulthood. Brain maturation includes changes in morphology and in composition. Changes in brain morphology consist of increase in brain volume and weight, developing sulcation, changes in ventricular shape, and decrease in volume of the subarachnoid spaces. These changes are well depicted with fetal imaging. From midgestation to infancy, brain growth reflects synaptogenesis, dendritic arborization and spine formation, axonal elongation and collateral formation, myelination, gliogenesis, neurotransmitter development, and vascular development. The most rapid changes occur between midgestation and the end of the second postnatal year, with 2 partially overlapping stages: the first is a period of oligodendrocytic proliferation and differentiation, whereas the second is a period of rapid myelin synthesis and deposition. Myelination progresses in a caudorostral way, at different times and speeds depending on specific fiber tracts, and at variable speeds within a given functional unit. Because the rate of myelination in a particular pathway may change with time, the onset of myelination before or at birth is not necessarily associated with early myelin maturation. Motor and sensory tracts mature early compared with association fibers. As a consequence in the forebrain, myelination progresses from the somatosensory central sulcus toward all poles but also from the visual occipital lobe and the auditory Heschl gyrus: the occipital pole myelinates before the frontal pole, which in turn myelinates before the temporal pole. This progression implies that lesions that affect the processes of myelination have a different distribution from diseases that affect the already formed myelin.
The immature brain is more prone to seize than the mature brain. The immature neurons and networks generate periodic discharges. In addition, gamma aminobutyric acid (GABA) exerts a paradoxic excitatory action in the immature brain that increases excitability. Maturational changes in excitatory amino acid (EAA) neurotransmission occur during the early postnatal period with consequent susceptibility to hypoxic seizures. Excessive release of EAA neurotransmitters, especially glutamate, mediates seizure activity and hypoxoischemic neuronal damage.
How to image
US, cranial CT, and MR imaging are used extensively for the diagnosis of neonatal brain disorders. In contrast, positron emission tomography (PET) is not commonly used in the neonatal period.
US
Cerebral US, whether in combination with Doppler technique or not, is an easily available modality that involves minimal or no disturbance to the neonate, even those who are sick and asphyxiated. However, cranial US has a low sensitivity, especially in term babies with hypoxic-ischemic injury. The signs for early detection of hypoxic-ischemic insult are small ventricles and subarachnoid spaces and increased echogenicity. However, it is not possible to determine whether an increased echogenicity reflects edema that may be transient, or permanent cell injury that will turn into a cystic lesion. Moreover, deep structures (brainstem, cerebellum) as well as superficial structures (cortical ribbon) are not easily imaged by cranial US ( Fig. 1 ). US is reported to be normal in as many as 50% of neonates with hypoxic-ischemic injury. It is also sometimes difficult to differentiate an ischemic stroke from a hemorrhagic infarction in the early stage because the echogenicity is similar. However, cranial US has improved significantly and is still the primary imaging modality in neonates for detecting intraventricular hemorrhages (IVH), hydrocephalus, and white matter changes. Periventricular leukomalacia (PVL) is suspected in cases of periventricular hyperechogenicity, but this may be confused with edema that can resolve without leaving residual damage. Normal sonograms have been reported in children proved to have PVL at autopsy. In very small premature babies (from 24 to 32 weeks), the white matter displays a diffuse hyperechogenicity, probably because of the interfaces that result from the multilayered pattern of the cerebral parenchyma (from outside to inside: the cortical ribbon, the subcortical white matter, the layers of migrant cell, the subventricular zone, and the germinal matrix; Fig. 2 ). Although nodules of leukomalacia can be identified easily on US, persistent hyperechogenicities or asymmetry of echogenicity suggest white matter damage without being specific. MR imaging is therefore the next step to consider because it is more sensitive and has a prognostic value in severe cases (neonates without respiratory autonomy, maternofetal infection). This step helps therapeutic decision making because 30% to 50% of premature infants with normal US present with white matter abnormalities on MR imaging. MR imaging is also the next step when cranial US does not explain the clinical state of a compromised neonate.
Midline malformations are easily depicted by US ( Fig. 3 ), but with less precise information compared with MR imaging.
Cranial CT
Modern CT is fast and versatile, and easily available. However, it uses X-rays, and radiation is deemed to be harmful, especially in young children. CT is usually performed without iodine injection, except in cases of neonatal meningitis, to look for complications such as empyema and thrombophlebitis. CT vascular imaging can also be performed, when necessary, because it is considered the standard for venography.
The normal neonatal brain is characterized by a low attenuation of the white matter compared with the cortex and basal ganglia ( Fig. 4 ). It is sensitive for detecting hemorrhage, HIE, and stroke ( Fig. 5 ), as well as the complications of birth trauma. In contrast, white matter damage and neuronal necrosis are more difficult to identify on CT, although the loss of gray-white contrast and the so-called laminar necrosis in the cortex show up well. Because of the low density of the white matter, it is difficult to detect nonhemorrhagic leukomalacia. Although CT is able to show cytotoxic edema by showing the effacement of the gray-white contrast (see Fig. 5 B), it still lacks the sensitivity of MR in cases of HIE in term newborns. Midline malformations such as complete agenesis of commissures and posterior fossa cyst are easily identified. CT also efficiently depicts brain swelling, particularly the swelling seen in aminoacidopathies such as leucine toxicity ( Fig. 6 ). However, MR imaging is better for optimally assessing malformations and inborn metabolic disorders. Epileptogenic tumors are not commonly detected in the neonatal period ( Fig. 7 ).
MR Imaging
MR is the most sensitive and, with the use of DWI, DTI, susceptibility imaging (SWI), MR spectroscopy, MRA, and MR venography, the most versatile modality to investigate the neonatal brain.
- •
Conventional MR imaging is still extremely helpful. Given the brain immaturity, optimal MR imaging parameters have to be chosen: gradient echo and inversion recovery T1-weighted images (T1WI) give excellent contrast between gray and white matter, and are highly sensitive to hemorrhage, calcifications, and neuronal necrosis. The recovery time (TR) and echo time (TE) of T2-weighted images (T2WI) have to be long enough in the neonate to adapt to the prolonged gray and white matter T1 and T2 relaxation times. Single-shot T2WI (ie, HASTE) is not efficient enough in evaluating neonatal tissular changes: it may provide anatomic information, but brain maturation and brain damage are not well depicted. Fluid attenuated inversion recovery (FLAIR) images are usually not used in neonates except to identify cysts. Gadolinium is not commonly given in neonates except in cases of meningitis, tumor, or vascular malformation.
The normal appearance of the neonatal brain is different ( Fig. 8 ) from the mature brain. Water content is high, especially within the white matter, and is responsible for the low signal on T1WI and bright signal on T2WI compared with gray matter. In contrast, basal ganglia show low signal on T2 and bright signal on T1 because of the high cell density. Areas that are already even partially myelinated show low signal on T2 and bright signal on T1: areas of primary cortex (central sulcus, hippocampus, Heschl gyrus, calcarine sulcus), posterior brainstem, optic tracts, and white matter underlying the central area. Posterior limbs of internal capsules (PLICs) display bright signal on T1 but are not yet fully myelinated. This latter appearance is present from 33 weeks on. In premature babies of less than 30 weeks, the brain tissular layering is apparent: migrating cells form waves within the white matter and the germinal matrix is thick, giving a multilayered pattern of the cerebral mantle. In high premature neonates, gyration has not yet developed, resulting in a lissencephalic appearance, although with a normal cortical thickness (see Fig. 2 ).
- •
DWI seems to be more sensitive than standard MR imaging in the detection of acute brain damage related to hypoxic-ischemic injury in the first day after the hypoxic event and in excitotoxic damage seen in some inborn errors of metabolism. Echo planar (EP) diffusion images are routinely and easily performed in the neonate, with an acquisition time of 1.0 to 1.30 minutes. Sensitization gradients are applied following the 3 axes (x, y, z). Generated images consist of trace images and maps of apparent diffusion coefficient (ADC). DWI is also sensitive to changes in cell density and myelination and shows signal changes before T1 and T2 sequences. In the neonate ( Fig. 9 ), ADC is lower in the hindbrain compared with the forebrain. The process of myelination is identified in the corticospinal tracts as a bright signal on DWI and low signal on ADC within the pons, the central area, and the posterior limb of the internal capsules. This pattern is also seen in the optic radiations and the splenium of the corpus callosum. Unmyelinated white matter shows low signal on DWI and high signal on ADC. Pathologic restriction of diffusion is seen in anoxic-ischemic ischemic disease with cytotoxic edema (stroke, HIE, mitochondrial disorders), in metabolic disease with vacuolating myelinopathy, or in conditions in which an increased cellular density occurs, such as inflammation or pus collection. In contrast, lesions with increased diffusivity, such as vasogenic edema, display normal or low signal on diffusion images and high signal on ADC maps.
Fig. 9
Normal appearance of term infant with DWI ( A – C ) and ADC maps ( D – F ). ADC is lower in the posterior fossa compared with cerebral hemispheres. Note process of myelination within the corticospinal tracts as bright signal on DWI and low signal on ADC within the pons, the central area, and the posterior part of the internal capsules. Unmyelinated white matter shows increased diffusivity.
- •
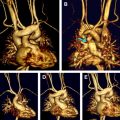
DTI evaluates the changing microstructure of the developing brain. Diffusion of water in the white matter becomes more restricted and gains anisotropy, which depends on the structural fascicular organization. Data are obtained from acquisitions made after applying diffusion gradients in multiple directions (at least 6 noncollinear). Acquisition time of this type of sequence is from 3 to 4 minutes with 6 directions, 5 to 6 minutes with 12 directions, and even longer with 20 directions when the pixels are isotropic. Shorter acquisition time is obtained with nonisotropic pixels. The images generated include trace images, ADC maps, fractional anisotropy (FA) maps, and color-coded orientation tractography maps ( Fig. 10 ). Color-coded orientation tractography maps are useful in the assessment of focal cortical dysplasia (FCD; Fig. 11 ) or tumor ( Fig. 12 ) in neonates by showing the disorganized tissue compared with conventional T1 and T2 images. White matter parcellation allows the assessment of FA ADC in a specific tract. However because of low FA in neonates, the tractography postprocessing tool needs be modified from what is used in the mature brain. Tractography is also used to investigate brain connectivity such as parahippocampal connectivity, corticothalamic connectivity, and motor connectivity. DTI studies in epileptic patients have shown increased diffusivity and decreased FA within and beyond the epileptic zone. However, no study is available in the neonate.
Fig. 10
DTI, FA map ( A ), and color-coded orientation map ( B ) of a healthy neonate.
Fig. 11
FCD, axial T1 ( A ) and T2 ( B ) images, and FA color map ( C ). Note the poor differentiation between cortex and white matter within the right frontal area ( A ) with low signal on T2 ( B ). FA color map shows the disorganized tissue compared with T1 and T2 images.
Fig. 12
Low-grade tumor (same patient as Fig. 7 ), trace image ( A ), ADC map ( B ), and FA color map ( C ). Focal lesion of the right amygdala shows slightly restricted diffusion. Note the disorganized tissues on the FA color map.
- •
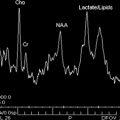
Proton MR spectroscopy can also be used to obtain complementary information on the brain status, especially in neonatal encephalopathies. Spectra may be acquired with the single-voxel technique or with chemical shift imaging (CSI) from 1 slab (two-dimensional CSI) or from multiple slices (three-dimensional CSI). Because of its robustness, position resolved spectroscopy sequence is commonly used, with short (30–35milliseconds) and long echo time (145 milliseconds). The neonatal brain is characterized by high concentration of choline (Cho), myo-inositol-glycine (mI), and glutamine plus glutamate (Glx) in relation to creatine (Cr) and N -acetylaspartate (NAA). Brain maturation is characterized by an increase of NAA and Cr, and a concomitant decrease of Cho, mI, and lipids. Inositol is a precursor molecule for inositol-lipid synthesis and is considered as an osmolyte and an astrocyte marker. Myo-inositol is the predominant peak from 22 to 28 weeks, and probably reflects the high density of glial cells that multiply and differentiate before myelinogenesis starts. The choline peak represents high levels of substrate needed for the formation of cell membranes, with gradual reduction as soon as incorporation of lipids has developed. NAA is considered a neuronal marker but is also expressed in oligo-type 2 astrocyte progenitors, immature oligodendrocytes, and mature oligodendrocytes. Therefore, NAA also reflects oligodendrocyte proliferation and differentiation. As the neuronal cell density decreases with dendritic maturation in the cortex, the increase in NAA with age may reflect a contribution of nonneuronal origin. Creatine reflects energy metabolism and has been shown to increase before and around term, and postnatally.
Regional variations are pronounced at all ages between gray and white matter, and also within different areas of gray and white matter. The highest choline, creatine, and NAA peak intensities are found in the thalami, followed by basal ganglia, and then other regions in preterm and term infants. This probably reflects the high cellular density in these structures and their more advanced maturation compared with white matter. Concentration of NAA and creatine is higher in gray matter than in white matter ,whereas choline is slightly lower. The reason for this is unclear, but it may be that gray matter contains fewer myelin membrane. For white matter, NAA and choline peak intensities are higher in the parietooccipital area than in the frontal white matter (the parietal area is myelinated before the frontal area, so the adult pattern is reached first in the parietooccipital region). The hindbrain has a peculiar metabolic pattern. The developing cerebellum shows a rapid NAA increase from infancy to childhood, and a rapid increase in creatine and Glx from fetal age, infancy, and childhood. The cerebellum has the highest concentration of Cr and is also characterized by high contents of Glx, choline, and myo-inositol-glycine compared with the cerebral hemisphere. Regional variations are also observed: the lowest concentrations are in the vermis, whereas the highest concentrations are in the pons. MR spectroscopy in epileptic patients has been widely used to delineate the epileptic zone, and has shown a decrease of NAA, especially in temporal lobe epilepsy and frontal lobe epilepsy. However, there is currently no available literature addressing the epileptic neonate.
Nuclear Medicine
Single-photon emission CT (SPECT) with technetium-99m-hexamethylpropyleneamine (99mTc-HmPAO) is widely used in neonatal seizures. SPECT relies on increased perfusion during the ictal period to detect an active epileptic focus. The increased perfusion of the epileptic focus is the result of the metabolic demands placed on the cerebral tissue during an epileptic seizure. Clinical and electrical seizures in neonates are associated with a focal cerebral hyperperfusion of the same amount as in adults. Although to a lesser degree than in adults, focal cerebral hyperperfusion in neonates corresponds with clinical seizures.
PET is a noninvasive imaging modality that has proved to be a powerful diagnostic tool for detecting neurochemical abnormalities associated with neurologic diseases. Although it has been well described in adults and pediatrics, its application in the newborn has not been extensively explored. Early detection of brain injury secondary to intrauterine and perinatal insults using PET imaging can provide new insights for instituting early therapy. The most common tracer used in the imaging of epilepsy for clinical purposes is 2-deoxy-2-[ 18 F]fluoro- d -glucose (FDG). FDG PET scans can detect areas of hypometabolism consistent with epileptogenic foci even in patients with normal MR imaging. In the neonate, when seizures are poorly controlled and there is no obvious cause for the seizures, the most likely causes are malformation of cortical development or an inborn error of metabolism. In this setting, if the MR imaging is not diagnostic, PET scanning with FDG may help in depicting an epileptic focus. If a diffuse pattern of abnormality is shown on the PET scan, a metabolic disorder is more likely.
How to image
US, cranial CT, and MR imaging are used extensively for the diagnosis of neonatal brain disorders. In contrast, positron emission tomography (PET) is not commonly used in the neonatal period.
US
Cerebral US, whether in combination with Doppler technique or not, is an easily available modality that involves minimal or no disturbance to the neonate, even those who are sick and asphyxiated. However, cranial US has a low sensitivity, especially in term babies with hypoxic-ischemic injury. The signs for early detection of hypoxic-ischemic insult are small ventricles and subarachnoid spaces and increased echogenicity. However, it is not possible to determine whether an increased echogenicity reflects edema that may be transient, or permanent cell injury that will turn into a cystic lesion. Moreover, deep structures (brainstem, cerebellum) as well as superficial structures (cortical ribbon) are not easily imaged by cranial US ( Fig. 1 ). US is reported to be normal in as many as 50% of neonates with hypoxic-ischemic injury. It is also sometimes difficult to differentiate an ischemic stroke from a hemorrhagic infarction in the early stage because the echogenicity is similar. However, cranial US has improved significantly and is still the primary imaging modality in neonates for detecting intraventricular hemorrhages (IVH), hydrocephalus, and white matter changes. Periventricular leukomalacia (PVL) is suspected in cases of periventricular hyperechogenicity, but this may be confused with edema that can resolve without leaving residual damage. Normal sonograms have been reported in children proved to have PVL at autopsy. In very small premature babies (from 24 to 32 weeks), the white matter displays a diffuse hyperechogenicity, probably because of the interfaces that result from the multilayered pattern of the cerebral parenchyma (from outside to inside: the cortical ribbon, the subcortical white matter, the layers of migrant cell, the subventricular zone, and the germinal matrix; Fig. 2 ). Although nodules of leukomalacia can be identified easily on US, persistent hyperechogenicities or asymmetry of echogenicity suggest white matter damage without being specific. MR imaging is therefore the next step to consider because it is more sensitive and has a prognostic value in severe cases (neonates without respiratory autonomy, maternofetal infection). This step helps therapeutic decision making because 30% to 50% of premature infants with normal US present with white matter abnormalities on MR imaging. MR imaging is also the next step when cranial US does not explain the clinical state of a compromised neonate.