Fig. 11.1
Transitional cell carcinoma. IVP shows large filling defect in the left side of the bladder (arrow). Confirmed on postoperative histological evaluation
IVP has a low sensitivity of 25 % for the detection of bladder neoplasms less than 5 mm in diameter.
Ultrasonography
On ultrasound, bladder tumors appear as echogenic structures protruding into the anechoic bladder lumen or local wall thickening (Figs. 11.2 and 11.3).
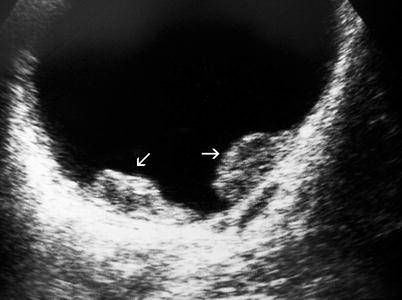
Fig. 11.2
Transitional cell carcinoma. Gray-scale ultrasound shows two solid papillary masses protruding into the bladder lumen (arrows)
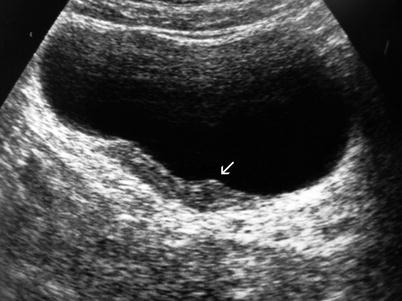
Fig. 11.3
Transitional cell carcinoma. Gray-scale ultrasound shows focal bladder wall thickening (arrow)
Although a hematoma in the bladder lumen will have the same appearance, hematoma will change its position in the bladder when the patient turns right or left (Fig. 11.4), but tumor will not.
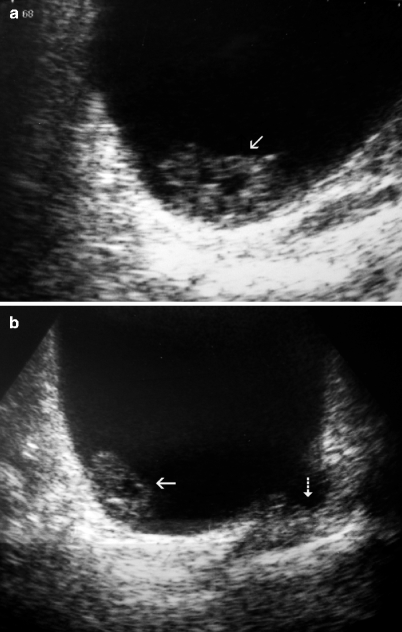
Fig. 11.4
Blood clot. (a) Gray-scale ultrasound shows a solid mass (arrow) at the posterior wall of the bladder. (b) The mass changes position when the patient turns right confirming it to be a blood clot (arrow in b). In addition, transitional cell carcinoma is also seen (dashed arrow in b)
Technically, the tumors located at the anterior wall of bladder are difficult to evaluate with ultrasound because of the reverberation artifacts.
Detection rates by ultrasound are significantly lower for lesions less than 5 mm in size. Presence of calcification in the tumor can be determined by ultrasound (Fig. 11.5). The presence of ureteral obstruction strongly suggests the presence of muscle invasion (Fig. 11.6).
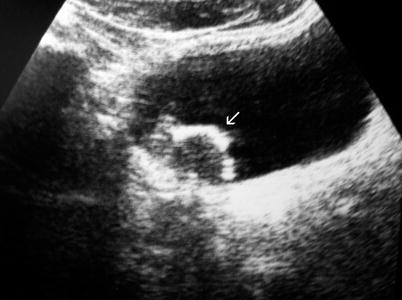
Fig. 11.5
Ultrasound reveals typical surface calcifications (arrow) of a bladder tumor
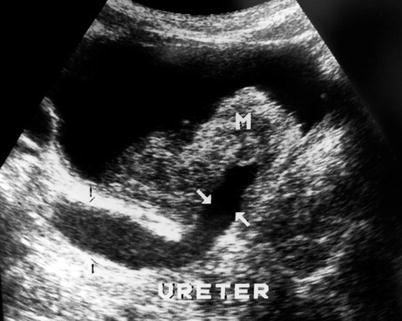
Fig. 11.6
Gray-scale ultrasound reveals a bladder tumor (M) which obstructs the right ureteric orifice and results in ureteral dilatation (arrows). This suggests the presence of muscle invasion
Bladder neoplasms located in the bladder neck area may be better detected with transrectal ultrasound (TRUS) (Fig. 11.7).
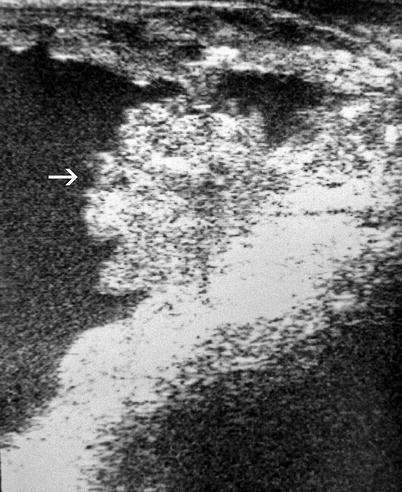
Fig. 11.7
Transrectal ultrasound; a large papillary bladder tumor (arrow) located at the bladder neck
Color flow Doppler ultrasound is helpful in eliciting blood flow within the bladder mass. Presence of blood flow within the mass does not correlate with stage or grade of tumor (Figs. 11.8 and 11.9).
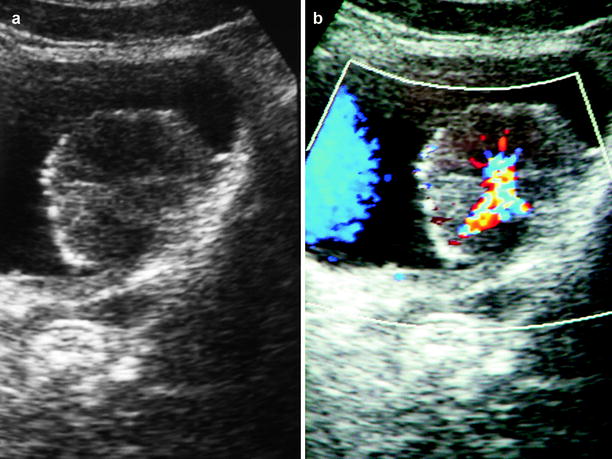
Fig. 11.8
Transitional cell carcinoma. (a) Gray-scale ultrasound shows a papillary tumor located on the posterior wall of the bladder. (b) Color flow Doppler ultrasound differentiates tumor from a blood clot (coagulum) by demonstrating blood flow within the mass
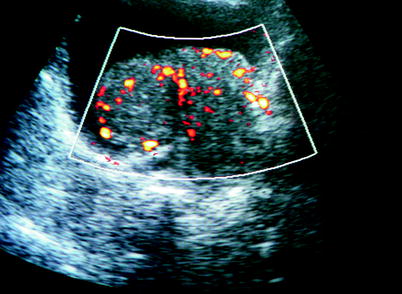
Fig. 11.9
Bladder tumor. Solid lesion located on the bladder base, intratumoral vascularization at power Doppler ultrasound aids in the differentiation of blood clot from bladder tumor
Three-dimensional imaging provides better spatial localization of bladder tumors (Figs. 11.10 and 11.11).
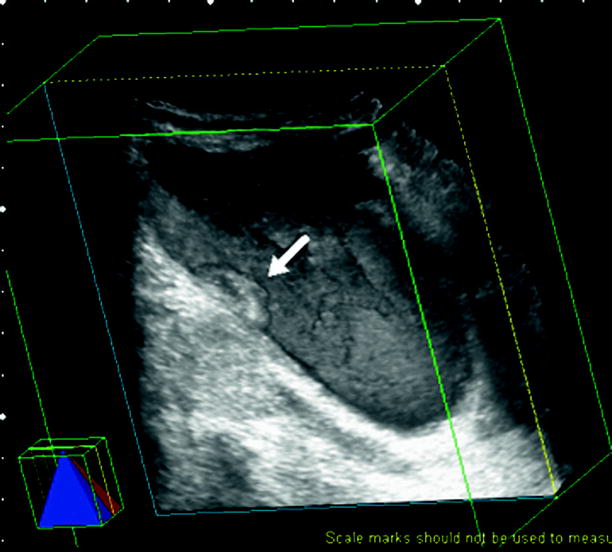
Fig. 11.10
Volume-rendered 3D ultrasound shows a polypoid bladder tumor (arrow) on the right lateral wall of the bladder (Courtesy of Ercan Kocakoç, M.D., Istanbul, Turkey)
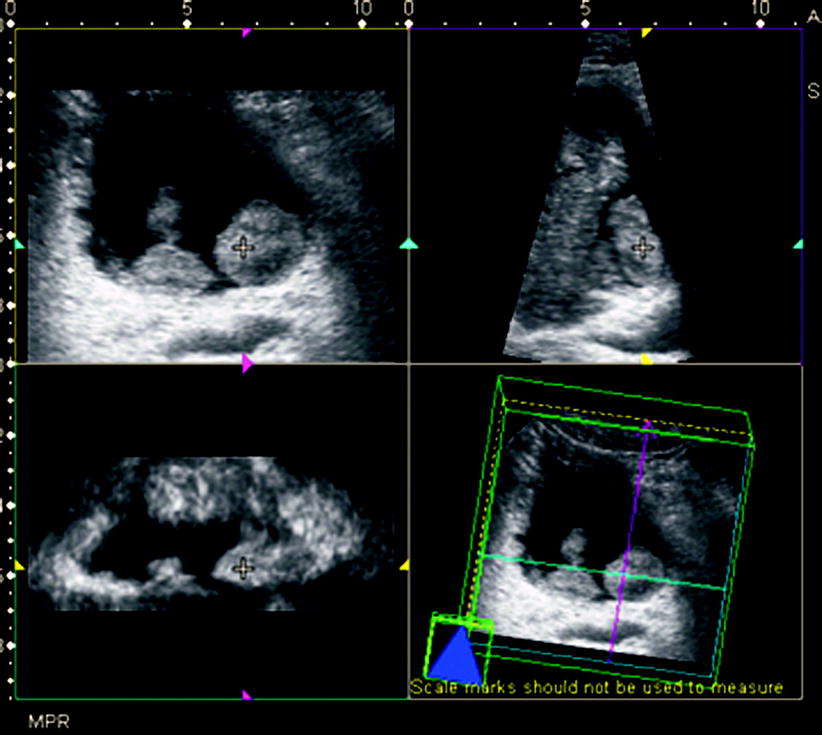
Fig. 11.11
Bladder tumor. Gray-scale ultrasound shows two polypoid lesions on the posterior wall of the bladder. Multiplanar reconstruction imaging (top left, transverse; top right, sagittal; and bottom left, coronal) shows lesions with poorly defined borders that are nearly identical to those seen on gray-scale images (Courtesy of Ercan Kocakoç, M.D., Istanbul, Turkey)
Computed Tomography
Overall reported accuracy of CT, for the detection of bladder tumors, varies from 60 to 95 %. CT helps in the recognition of local spread, lymphadenopathy, metastatic disease, and synchronous lesions in the bladder, ureter, and kidney.
On CT, urothelial carcinoma appears as intraluminal papillary or nodular masses or focal wall thickening (Figs. 11.12 and 11.13). In some cases, the intraluminal surface of the tumor may be encrusted with fine calcifications.
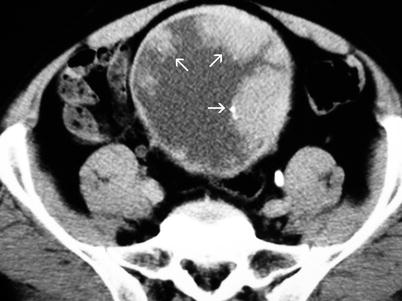
Fig. 11.12
Transitional cell carcinoma. Contrast-enhanced axial CT shows enhancing papillary lesions (arrows) with fine surface calcifications, located on the anterior and lateral wall of the bladder
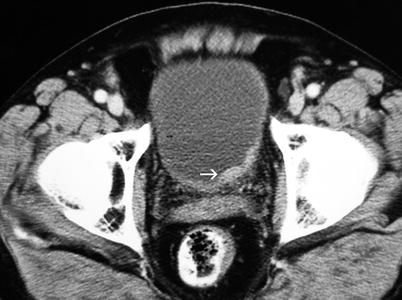
Fig. 11.13
Transitional cell carcinoma. Contrast-enhanced axial CT shows focal bladder wall thickening (arrow), with enhancement
The nephrographic phase (100–120-s delay) is the best for the evaluation of bladder tumor enhancement pattern (Fig. 11.14).
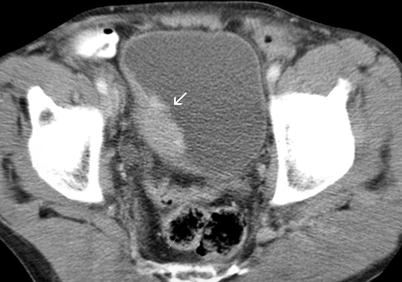
Fig. 11.14
Transitional cell carcinoma. Axial contrast-enhanced CT through bladder demonstrates enhancing right lateral bladder wall tumor (arrow)
Computed tomography cystography is usually used to assess bladder trauma but can be used in the evaluation of bladder tumors. It is performed by instillation of contrast material into the bladder lumen via urethral or suprapubic catheter.
The tumoral mass can be detected as soft tissue filling defect in the contrast material-filled hyperattenuating lumen on CT cystogram (Figs. 11.15 and 11.16).
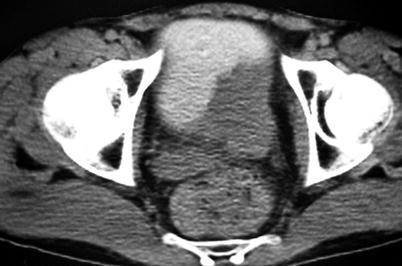
Fig. 11.15
Bladder tumor. Late-phase contrast-enhanced axial CT; tumor on the left posterior wall seen as a filling defect in the contrast material-filled bladder lumen
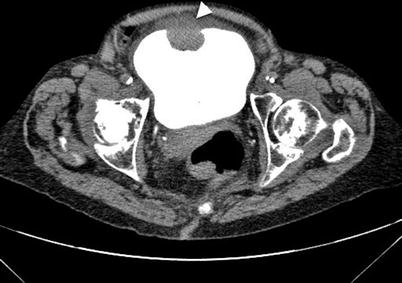
Fig. 11.16
Bladder tumor on CT cystogram. CT cystogram image obtained after filling of bladder lumen with contrast material demonstrates a mass lesion in the anterior wall of bladder with extension in to the extravesical fat tissue (arrowhead)
Tumoral invasion of perivesical tissues can be demonstrated in addition to intraluminal tumor involvement on CT cystography (Fig. 11.16).
Unfortunately, it is impossible to resolve the bladder wall layers as lamina propria and muscle or to differentiate between the superficial and deep muscle layers despite the development of current CT techniques. Consequently, CT cannot accurately determine the depth of bladder wall invasion, i.e., differentiation of stage T1 from stage T2 or differentiation of stage T2a from stage T2b disease, but it can distinguish stage T3b or higher stage tumors with reported accuracies of 83–93 %.
Stage T3b tumors result in an irregular outer bladder wall abnormality or soft-tissue infiltration or stranding into the perivesical fat (Fig. 11.17).
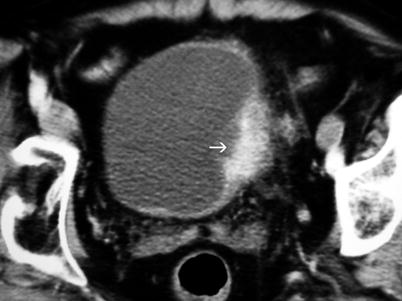
Fig. 11.17
Bladder tumor. Contrast-enhanced axial CT reveals enhancing tumor (arrow) on the left lateral wall of the bladder with perivesical stranding and wall irregularity; suspected perivesical invasion
In the presence of invasion into adjacent organs, the tumor is classified as stage T4 disease (Fig. 11.18).
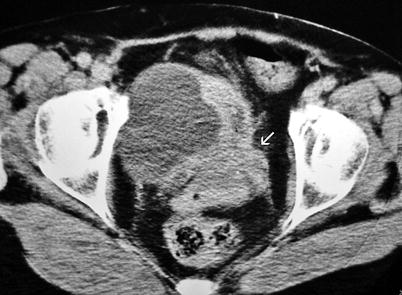
Fig. 11.18
Axial contrast-enhanced CT through bladder demonstrates invasive bladder carcinoma located on the base of the bladder with cervical and vaginal invasion (arrow)
CT cannot distinguish between reactive or neoplastic perivesical infiltration.
Magnetic Resonance Imaging
The accuracy of MRI in the staging of bladder cancer is reported to be 10–33 % higher than those obtained on CT. In general, the reported accuracy of MRI in overall staging varies from 60 to 90 %, whereas that of local staging varies from 73 to 96 %. The accuracy of gadolinium-enhanced MRI for staging extravesical extension is between 73 and 100 %.
CT and MRI are usually used to evaluate extravesical extension and/or to stage the tumor. Early microscopic extravesical invasion (stage T3a) cannot be reliably detected by CT or MRI. Late macroscopic extravesical extension of disease (stage T3b) is diagnosed by CT and MRI as an irregular ill-defined outer wall of the bladder with/without linear stranding or nodules. Importantly, an enhancing mass involving the bladder wall and adjacent structures strongly suggests the presence of the tumor invasion.
Dynamic MRI may be useful in the evaluation of bladder tumors with staging accuracy ranging from 60 to 90 %.
The tumor appears intermediate in signal intensity on T1-weighted MRI, and gadolinium-enhanced T1-weighted image shows an intense and immediate enhancement of the tumor tissue (Fig. 11.19). Typically, the tumor enhances earlier than the normal bladder wall due to neovascularity. The tumor tissue is intermediate in signal intensity, greater than the normal musculature, on T2-weighted MRI sequence (Fig. 11.20).
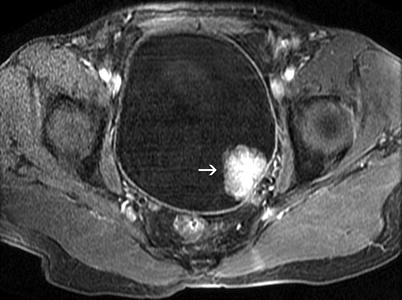
Fig. 11.19
Transitional cell carcinoma. Axial T1-weighted fat-saturated postcontrast MR image obtained with gradient echo sequence shows a polypoid mass lesion (arrow) arising from the posterolateral wall of the bladder. Bladder mass demonstrates marked enhancement and that the integrity of the muscular layer is preserved
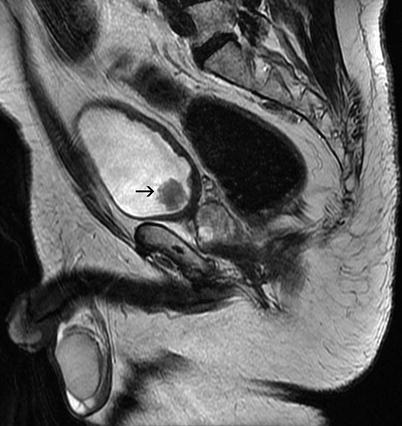
Fig. 11.20
Transitional cell carcinoma. Sagittal FSE T2-weighted MRI shows that lesion (arrow) has a low- to intermediate-signal intensity and that the muscular layer is not invaded by the tumor tissue
On T2-weighted MRI, the preservation of the low-signal bladder wall adjacent to the tumor tissue suggests the presence of a superficial tumor component, and any difference in the signal intensities between the inner and outer layers of the muscularis propria may help in the assessment of tumor depth (Figs. 11.21 and 11.22).
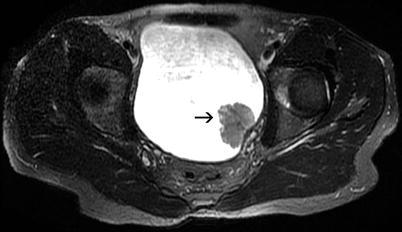
Fig. 11.21
Transitional cell carcinoma. Axial FSE fat-saturated T2-weighted MRI shows that the mass lesion (arrow) has a moderate intensity and the integrity of the muscular layer is preserved
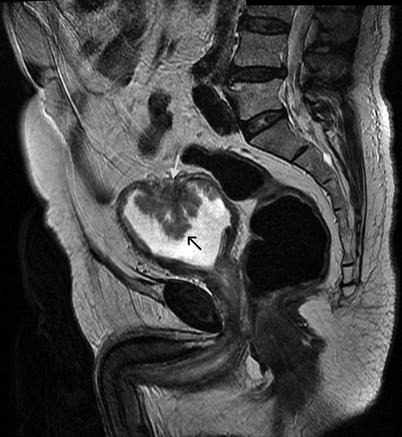
Fig. 11.22
Invasive bladder cancer (black arrow). Sagittal FSE T2-weighted MRI demonstrates discontinuity of the hypointense muscular layer (white arrow)
Invasion of the tumor into the surrounding visceral organs like prostate, uterus, or vagina is also better appreciated on T2-weighted and contrast-enhanced T1-weighted MRI sequences (Fig. 11.23).
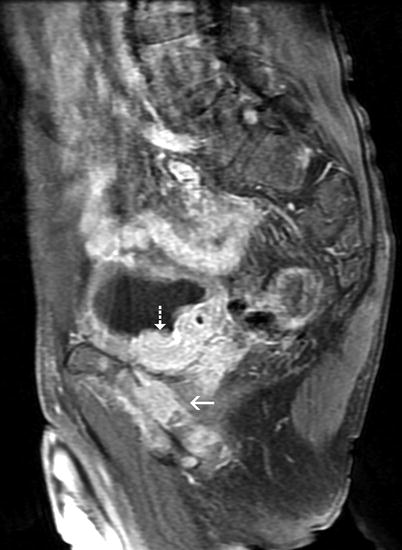
Fig. 11.23
Bladder tumor. Sagittal fat-saturated FSE T1-weighted postcontrast MRI. The markedly enhanced tumoral lesion (dashed arrow) located on the bladder base has extensive perivesical invasion and also involves the pubic bone (arrow). The tumor was assessed to be consistent with stage T4 disease
Virtual Cystoscopy Techniques
In patients with severe urethral strictures or in the presence of active bleeding, virtual cystoscopy is an alternate technique.
Virtual cystoscopy is limited in detection of flat tumors and differentiation of small tumors from mucosal edema or vesical muscle trabeculation.
Nuclear Scintigraphy
Nuclear scintigraphy is of limited value in evaluating the presence or absence of bone metastases in cases of invasive bladder carcinoma.
Characteristically, bone scintigraphy may demonstrate metastatic foci with increased or decreased activity.
Positron emission tomography (PET) may be useful in the lymph node staging and may also be useful in differentiating tumor recurrence from postradiation fibrosis. FDG-PET may also be used to detect distant organ metastases (Fig. 11.24).
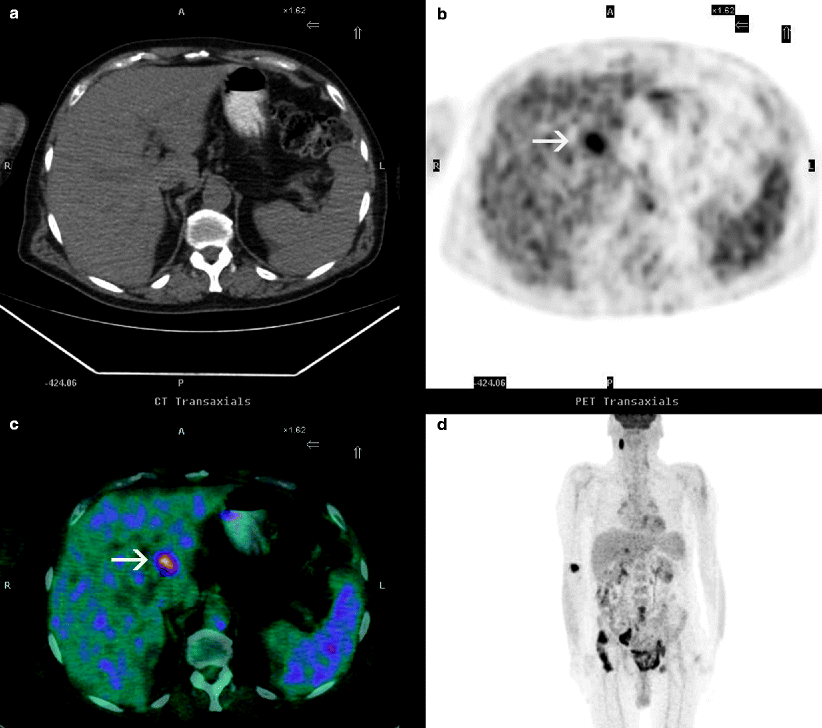
Fig. 11.24
Axial CT (a), PET (b), PET-CT (c), and coronal MIP (d) images of a 68-year-old patient with bladder carcinoma reveal a focus of intense activity in the liver (arrows) consistent with a metastatic disease
Pathology
Urothelial Carcinoma In Situ
Urothelial carcinoma in situ (CIS) is a flat intraepithelial carcinoma. CIS is characterized by flat disordered proliferation of urothelial cells with marked cytologic atypia and pronounced loss of cell polarity and cohesiveness (Fig. 11.25).
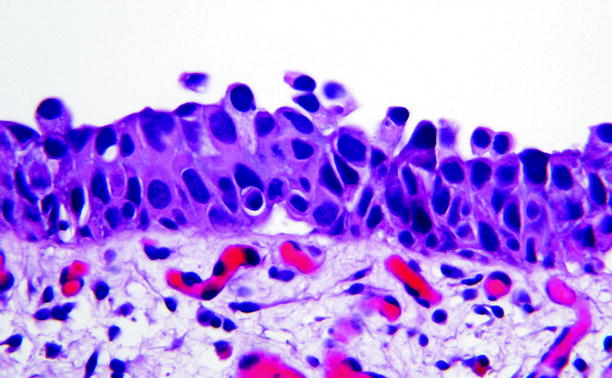
Fig. 11.25
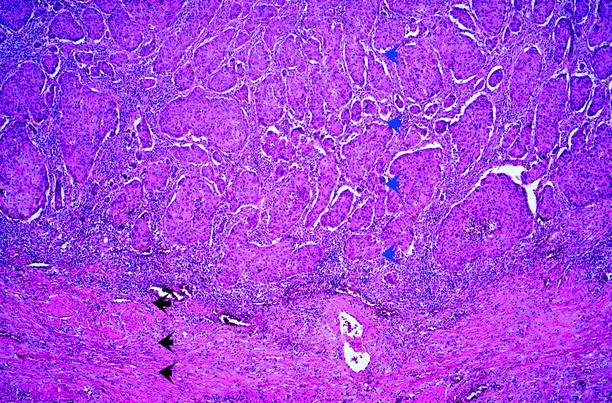
Carcinoma in situ. There is a disordered proliferation of malignant urothelial cells whose nuclei show a high nuclear/cytoplasmic ratio, nuclear pleomorphism, irregular nuclear contours, and hyperchromatic or coarsely granular chromatin. The cells lack polarity entirely. Vascular proliferation is often present in the underlying stroma. Large, prominent, and multiple nucleoli may be seen in at least some cells. Mitotic figures may be seen at any level and are often atypical. This is stage Tis (see Fig. 11.30)
Mitotic figures are abundant and are often seen in the uppermost urothelium. Urothelial CIS has a high likelihood of progressing to invasive carcinoma if untreated, an outcome that occurs in up to 83 % of cases.
Papillary and Invasive Urothelial Carcinomas
About 6,000 individuals develop bladder carcinoma annually in the United States, with urothelial carcinoma accounting for the overwhelming majority of these cases.
Papillary urothelial carcinoma is typically an exophytic tumor, which may appear solid or composed of numerous papillae, when viewed endoscopically (Fig. 11.26). Tumors may be solitary (60 %) or multiple (Fig. 11.27).
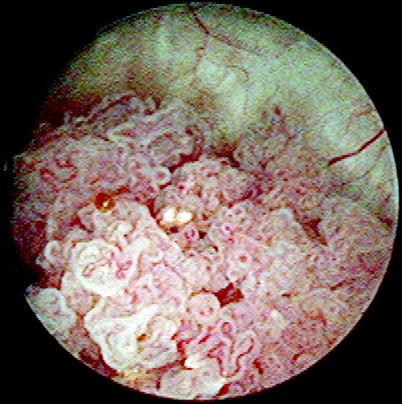
Fig. 11.26
Papillary urothelial carcinoma. Cystoscopic view shows an exophytic papillary growth. The delicate vasculature of the lesion is readily apparent
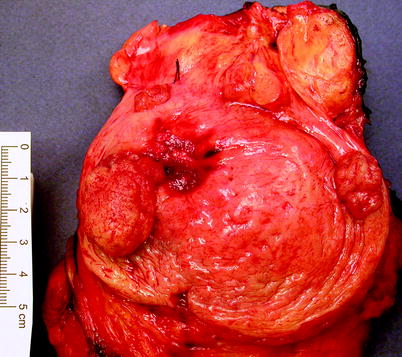
Fig. 11.27
Papillary urothelial carcinoma. These neoplasms are frequently multifocal. The tumors shown in this image were all noninvasive. Cystectomy was done because a muscle-invasive cancer had previously been resected from the area of hemorrhagic scarring
Papillary urothelial carcinoma is composed of malignant urothelial cells growing on the surface of papillary fibrovascular cores. The degree of cytologic atypia is variable and is used to assign a grade. Squamous or glandular differentiation is commonly present in portions of these tumors, particularly in those of higher grade.
Some urothelial carcinomas have little or no papillary component and are invasive from inception. Such cancers are often high grade and biologically aggressive, and they typically produce a prominent desmoplastic stromal reaction. Many of the variants of urothelial carcinoma (nested, micropapillary, lymphoma-like, sarcomatoid) fall within this category.
Staging Urothelial Carcinoma
Tumor staging is critical in predicting the disease course of an affected individual (Figs. 11.28, 11.29, 11.30, 11.31, 11.32, 11.33, 11.34, 11.35, 11.36, 11.37, and 11.38).
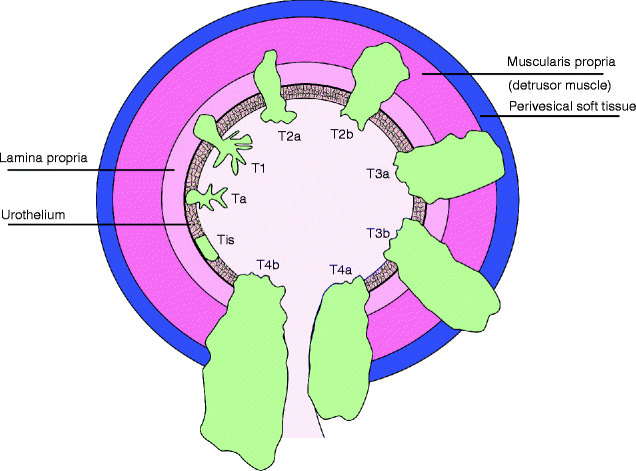
Fig. 11.28
Papillary urothelial carcinoma. Graphic representation of the stages of urothelial carcinoma (From Cheng L, Lopez-Beltran A, MacLennan GT, Montironi R, Bostwick DG. Neoplasms of the urinary bladder. In: Bostwick DG, Cheng L, editors. Urologic surgical pathology. 2nd ed. Edinburgh: Mosby/Elsevier; 2008, with permission)
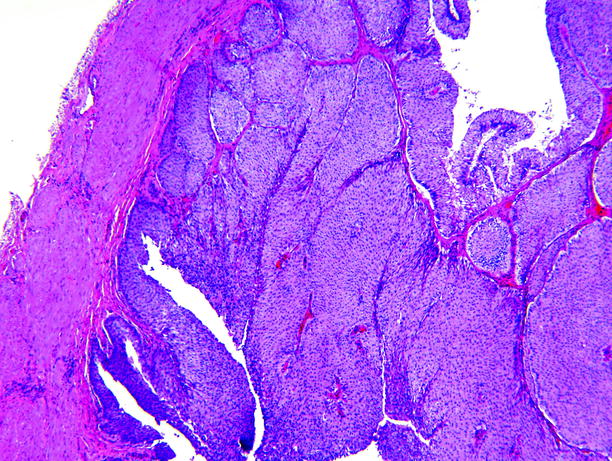
Fig. 11.29
Papillary urothelial carcinoma. An exophytic papillary neoplasm rests upon the muscle, at left. No stromal invasion is present. This is stage Ta
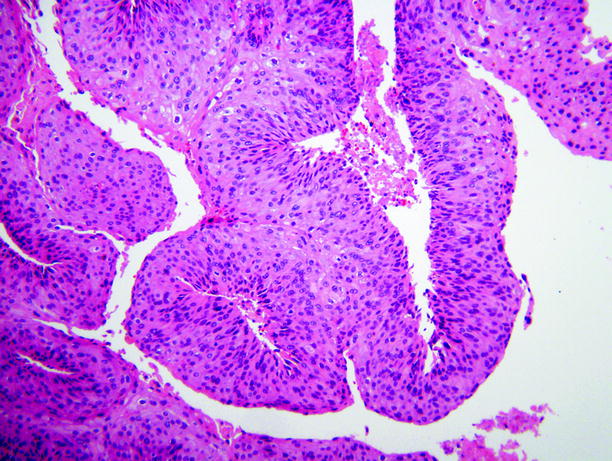
Fig. 11.30
Papillary urothelial carcinoma. This is a low-grade carcinoma. The nuclei lack uniformity of shape and size and the tumor cells exhibit loss of cellular orientation
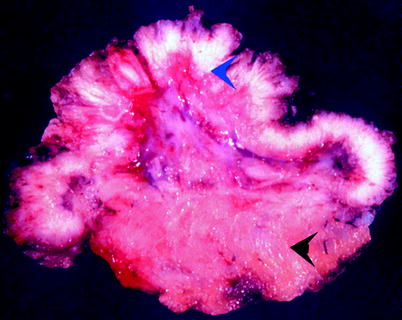
Fig. 11.31
Papillary urothelial carcinoma. This low-grade carcinoma is focally invasive into lamina propria (blue arrowhead). The detrusor muscle (black arrowhead) is uninvolved by tumor. This is stage T1 (From MacLennan GT, Resnick MI, Bostwick DG. Pathology for urologists. Philadelphia: WB Saunders; 2002, with permission)