Neoplastic Abnormalities
Sathish Kumar Dundamadappa
Lidia Mayumi Nagae
Prachi Dubey
QUESTIONS
1 A 55-year-old male presents for further workup of a right frontal lobe calcified mass incidentally discovered on CT for altered mental status.
|
1a What is the most likely diagnosis?
A. Meningioma
B. Oligodendroglioma
C. Metastasis
D. Primitive neuroectodermal tumor
View Answer
1a Answer B. Oligodendroglioma. The most appropriate choice would be oligodendroglioma. A meningioma is typically an extra-axial dural-based lesion. A metastasis will be an appropriate differential possibility; however, a metastatic lesion of this size and in such a location is likely to incite more vasogenic edema (note that it is possible for a metastatic lesion to not incite large amount of vasogenic edema, typically when a lesion is present peripherally in the cortical or juxtacortical location).
1b Which of the following is a rare manifestation of oligodendroglioma?
A. Involvement of cortical/subcortical white matter
B. Presence of coarse intratumoral calcification
C. None or subtle enhancement on postcontrast MRI
D. Intraventricular location
View Answer
1b Answer D. Intraventricular location. The presence of an intraventricular oligodendroglioma is possible but rare, approximately 3% to 8%. These tumors when present have different imaging features compared to parenchymal oligodendrogliomas; for example, presence of enhancement, tumor blush on angiography, and high attenuation on CT relative to brain parenchyma are seen with these lesions but not with parenchymal oligodendroglioma. The other more typical intraventricular neoplasms are meningioma, central neurocytoma, ependymoma/subependymoma, and choroid plexus papilloma.
|
Imaging Findings: Noncontrast head CT showing calcifications within a right frontal lobe cortical based mass (A) and contrast enhanced T1w MRI in coronal plane showing heterogenous enhancement (B). This location and calcification are typical for oligodendroglioma.
Discussion: Oligodendroglioma is a tumor of neuroglial origin with variable prognostic features, ranging from relatively low aggressive potential relative to a similar-grade astrocytoma to very aggressive behavior similar to higher grade glial neoplasms. It is the third most common glioma, accounting for 5% to 18% of all glial neoplasms.
Calcification within oligodendroglioma may range from 20% to 90%; occasionally cystic degeneration and hemorrhage may also be seen. On MR, most commonly heterogenous signal intensity is noted; vasogenic edema although present is typically not as striking or common as the higher grade astrocytoma, and the ADC values on diffusion-weighted imaging might be low, such as seen with other higher grade glial neoplasm, purported to have resulted from lowered extracellular hyaluronic acid.
Differential Diagnosis: Other primary CNS glial neoplasm, metastasis, or less likely meningioma. Although other primary CNS glial neoplasms and metastasis cannot be excluded, the presence of calcification, peripheral cortical location, and lack of significant vasogenic edema are most typical for oligodendroglioma. Meningioma is not likely because this is a peripheral but intra-axial lesion instead of an extra-axial lesion.
References: Koeller KK, et al. From the archives of the AFIP: oligodendroglioma and its variants: radiologic-pathologic correlation. Radiographics 2005;25(6):1669-1688.
Smith AB, et al. From the radiologic pathology archives: intraventricular neoplasms: radiologicpathologic correlation. Radiographics 2013;33(1):21-43.
2 A 67-year-old hypertensive otherwise healthy man presents with new-onset seizure to the emergency department. A noncontrast head CT was performed to evaluate for intracranial hemorrhage.
|
2a What should be the next step?
A. Normal; no further imaging is needed
B. CT angiogram of head and neck
C. MRI of brain without and with contrast
D. FDG PET/CT
View Answer
2a Answer C. MRI of brain without and with contrast. There is a heterogenous mass-like lesion in the left temporal lobe; the best way to assess this will be MRI with contrast because of higher soft tissue detail. FDG PET and CTA will not be appropriate modalities in this case.
A. Glial neoplasm, such as glioblastoma
B. Primary CNS lymphoma
C. Metastasis
D. Herpes encephalitis
View Answer
2b Answer A. Glial neoplasm, such as glioblastoma. The location, heterogeneous enhancement, and necrotic foci favor glioblastoma.
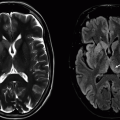
Imaging Findings: Figure 1. A mass-like cortical-based lesion in the left temporal lobe with necrotic/cystic foci on noncontrast head CT.
Figure 2. The same lesion on FLAIR (A), T2w (B), and contrast-enhanced T1w (C) sequences, with a heterogenously enhancing morphology, cystic/necrotic foci, and nonenhancing T2/FLAIR hyperintensity. These features are most consistent with a glial neoplasm with heterogenous enhancement pattern and potential necrosis favoring a relatively aggressive behavior.
Discussion: The above case shows an example of an aggressive primary CNS glial neoplasm, glioblastoma. It is the most common primary CNS neoplasm (˜15% of all intracranial neoplasms).
In distribution, it is frequently supratentorial, predominantly involving ages 40 to 70 years. When it is rarely found in children, it may be more commonly infratentorial compared to adults.
The majority of these lesions have a rapid clinical course, have an abrupt presentation, and arise de novo within the brain, that is, without a preexisting lower grade precursor lesion. However, a few lesions may result from de-differentiation of a preexisting lower grade glial neoplasm; typically, the latter is seen in younger population (mean age, 40 years) and has a longer clinical course.
Proton MR spectroscopy typically demonstrates elevation of choline (3.2 ppm) and lactate peak (1.3 ppm). MR perfusion (either DSC or DCE) shows elevated CBV within the lesion, as would have been the case with any other aggressive neoplasm and occasionally with aggressive demyelination/inflammation. However, it is noteworthy that both MR perfusion and proton MR spectroscopy, are nonspecific in isolation and should be interpreted in conjunction with lesion morphology on other sequences and clinical presentation.
Differential Diagnosis: Metastasis, primary CNS lymphoma, herpes encephalitis, are differential consideration; however as explained above, the most likely diagnosis will be primary CNS glial neoplasm.
There has been a recent update in the glial neoplasm classification as of June 2016 with incorporation of the specific molecular profile. This was necessary because the histopathologic classification alone did not accurately predict clinical course or treatment response. For example, presence of IDH mutation, and ATRX loss are associated with a favorable prognosis.
References: Altman DA, Atkinson DS Jr, Brat DJ. Best cases from the AFIP: glioblastoma multiforme. Radiographics 2007;27(3):883-888.
Brat DJ, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015;372(26):2481-2498.
Louis DN, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131(6):803-820.
3 A surveillance MRI performed for a known condition. Key images are shown below:
|
3a Which of the following is likely to occur in these patients?
A. Pancreatic neuroendocrine tumors
B. Plexiform neurofibroma
C. Port-wine stain
D. Renal angiomyolipoma
View Answer
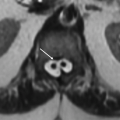
3a Answer A. Pancreatic neuroendocrine tumors. This is a case of von Hippel-Lindau syndrome with evidence of globe enucleation secondary to retinal hemangioblastoma, a left-sided endolymphatic sac tumor, and a right cerebellar hemangioblastoma. This entity has multisystem involvement with one of the manifestations being pancreatic neuroendocrine tumor (or islet cell tumor), seen in 5% to 17% of these patients.
|
Imaging Findings: Axial FLAIR sequence demonstrating a focus of hyperintensity in the right anterior cerebellum (A) with corresponding punctate enhancement seen on postcontrast T1w sequence (C) and a left petromastoid FLAIR hyperintense ovoid lesion, (A); there is enucleation of the left eye seen on axial T2w sequence (B). Constellation of findings suggest a right cerebellar hemangioblastoma, left endolymphatic sac tumor and left globe enucleation due to prior retinal hemangioblastoma, this is compatible with the diagnosis of von Hippel-Lindau syndrome.
Discussion: Hemangioblastoma is a benign slow-growing tumor of vascular origin. Its pathogenesis and cell of origin are not known. About two-thirds of these are sporadic, and the rest are seen in association with von Hippel-Lindau syndrome (VHL). Multiple tumors are almost always associated with VHL. A rare non-VHL form of multiple hemangioblastomas has been reported, known as leptomeningeal hemangioblastomatosis. Metastasis is extremely rare, but has been reported.
They are primarily seen in third to fifth decade of life; the presentation is earlier by a decade in VHL-associated tumors. They account for about 2% of intracranial tumors and 10% of posterior fossa tumors. They are the most common primary brain tumor of the posterior fossa in an adult. Most are located in the posterior fossa (cerebellum, vermis, and medulla in that order), followed by the spinal cord. Supratentorial location is rare, seen in about 5% to 10%. The growth pattern can be “stuttered” with periods of quiescence and growth.
The most common tumor morphology is peritumoral cyst with an intensely enhancing tumor nodule, seen in about 60%. About 30% of tumors are purely solid. Solid tumors, intratumoral cysts, and tumors with intra- and peritumoral cyst are rare patterns.
On CT, the cyst is hypodense and the nodule is almost isodense to the parenchyma. On MRI, the cyst shows fluid signal on T2 images. The solid nodule shows moderate hyperintensity on T2 and hypointensity on T1. Intense contrast enhancement of the nodule is typical. Tortuous vessels can be demonstrated in the vicinity of the tumor in precontrast, postcontrast, and CTA/MRA images. The surrounding edema is variable and can be disproportionately large compared to the nodule size. In larger nodules and solid masses, the enhancement can be heterogeneous. The cyst wall either shows no enhancement or minimal thin enhancement.
Differential diagnosis for cerebellar hemangioblastoma:
In children, pilocytic astrocytoma.
In adults, metastatic disease. In patients with VHL, metastasis from renal cell cancer can be hypervascular and may pose diagnostic difficulty.
References: Naidich TP, ed. Imaging of the brain. Expert radiology series. Philadelphia, PA: Saunders/Elsevier, 2013.
Osborn AG. Osborn’s brain: imaging, pathology, and anatomy, 1st ed. Salt Lake City, UT: Amirsys Pub., 2013.
A. A contrast-enhanced CT scan
B. A contrast-enhanced MRI
C. CT angiogram of the head
D. No further follow-up evaluation is needed.
|
View Answer
4a Answer B. A contrast-enhanced MRI. Given there is an abnormality within the right IAC, best seen with obvious expansion of the IAC on bone window (Figure 1B), the best way to evaluate this will be an MRI with contrast.
4b What is the most likely diagnosis based on the contrast enhanced MRI performed on this patient?
A. Vestibular schwannoma
B. CP angle meningioma
C. Neurofibroma
D. Epidermoid lesion
View Answer
4b Answer A. Vestibular schwannoma. The most likely explanation for this enhancing mass without restricted diffusion will be a vestibular schwannoma. Neurofibroma, also a nerve sheath tumor, has greater risk of malignant transformation but is exceedingly rare as a primary intracranial lesion, even in patients with type 1 neurofibromatosis, making this a very unlikely diagnosis for CP angle masses. CP angle meningioma is a differential possibility and cannot be reliably excluded on imaging alone. However, meningioma typically presents with a dural tail and adjacent reactive osseous hyperostosis and may also have restricted diffusion due to high cellularity, not seen in this case. Epidermoid lesion typically follows CSF appearance on both CT and MRI; however, it also demonstrates restricted diffusion. Enhancement if any is typically peripheral in nature.
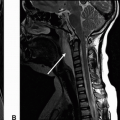
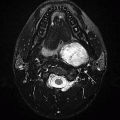
Imaging Findings: Figure 1. Axial noncontrast CT demonstrates a mass in the right cerebellopontine angle (CP angle) with extension into the right internal auditory canal (IAC). Note, on the bone windows (B), there is expansion of the right-sided IAC (it is key to carefully scrutinize the IAC on bone windows to detect any masses or space-occupying lesions that may remain occult on standard brain window).
Figure 2. A subsequent contrast-enhanced MRI confirms presence of an enhancing mass involving the right IAC and right CP angle, (A). No evidence of restricted diffusion to suggest high cellularity (C).
Discussion: Vestibular schwannoma is the most common mass in the CP angle, accounting for 85% of CP angle masses. Schwannoma is a benign, slowly growing neoplasm. CP angle schwannomas most commonly involve the inferior division of the vestibular nerve (vestibular branch of CN VIII). A majority of the intracranial schwannomas are sporadic. Less than 20% of these may be associated with neurofibromatosis type 2. Intracranial schwannomas most commonly occur along sensory nerve fibers, of which CN VIII is most common, followed by trigeminal nerve.
Patients commonly present with hearing loss, rarely with vertigo and imbalance. Management of such lesions can include observation, Gamma Knife surgery, or microsurgical resection. Various factors influence the treatment choices, and therefore further subspecialty consultation will be warranted to better understand treatment options.
Differential Diagnosis: Mengingioma (a dural tail, reactive hyperostosis in the underlying bone, and potentially restricted diffusion due to higher cellularity may be seen as differentiating features; however oftentimes this is difficult to definitively differentiate, particularly because meningiomas may have canalicular AND labyrinthine extension); epidermoid (follows CSF signal and shows restricted diffusion); metastasis (rare in isolation; typically seen with leptomeningeal disease, prior history of a primary).
References: Fisher JL, et al. Loud noise exposure and acoustic neuroma. Am J Epidemiol 2014;180(1):58-67.
Silk PS, et al. Surgical approaches to vestibular schwannomas: what the radiologist needs to know. Radiographics 2009;29(7):1955-1970.
Smirniotopoulos JG, et al. Cerebellopontine angle masses: radiologic-pathologic correlation. Radiographics 1993;13:1131-1147.
5 A 20-year-old male presents with gait disturbances and abnormal NECT. An MRI with contrast was performed for further assessment. Key images are shown below:
|
5a What is the most likely diagnosis?
A. Ependymoma
B. Subependymoma
C. Oligodendroglioma
D. Metastasis
5b These tumors originate from which of the following cell types?
A. Ependymal cells
B. Astrocytes
C. Oligodendrocytes
D. Cell type outside the CNS.
View Answer
5b Answer A. Ependymal cells. These tumors originate from the ependymal lining of ventricles and spinal cord central canal, hence the name “ependymoma”; however, it should be noted that extraventricular variants of this tumor have also been reported.
|
Imaging Finding: A calcified lesion on NECT (A) centered in the fourth ventricle with cystic foci on T2w sequences (F). There is no significant vasogenic edema on FLAIR (D) or evidence of restricted diffusion (E). Pre- and postcontrast images (B and C, respectively) show heterogeneous enhancement and elongated appearance that appears to squeeze through the foramen magnum on the sagittal plane, compressing the dorsal aspect of the cervicomedullary junction.
Discussion: Ependymoma is a type of primary CNS neoplasm that arises from the ependymal lining. They are within the subtype of neuroepithelial neoplasms unlike astrocytomas, which are primary neuroglial subtype of tumors.
These tumors can be seen at any age. Typically, younger patients tend to have posterior fossa and older patients tend to have supratentorial ependymoma. These can be found in the fourth, lateral, and third ventricles. These can also be extraventricular in location particularly when seen in a supratentorial location in children. These are classified as WHO grade II lesions. Variant of higher grade or anaplastic ependymomas have also been reported (WHO grade III).
Imaging features on MR are typically hyperintensity on T2-weighted images and isointensity on T1-weighted images with intense contrast enhancement within the soft tissue components. Calcification, hemorrhage, and cystic components are commonly seen that lead to morphologic heterogeneity within this lesion. These lesions are classically defined as soft or plastic lesions; when seen in the fourth ventricle, these can squeeze through the foramen of Luschka, foramen of Magendie, and foramen magnum.
Postoperative imaging is key in determining survival rate with residual disease associated with a lower overall survival.
References: Cage TA, Clark AJ, Aranda D, et al. A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. J Neurosurg Pediatr 2013;11(6): 673-681.
Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I. From the radiologic pathology archives: intraventricular neoplasms: radiologic-pathologic correlation. Radiographics 2013;33(1):21-43.
6 Provided below are key images from an MRI performed for a known intraventricular mass seen on CT.
|
6a What is the likely diagnosis for the lesion shown above?
A. Central neurocytoma
B. Ependymoma
C. Subependymal giant cell astrocytoma
D. Intraventricular metastasis
6b Which is the common anatomic site for these lesions?
A. Lateral ventricle attached to the septum pellucidum or ventricular wall
B. Choroid plexus in the fourth ventricle
C. Third ventricle
D. Commonly extraventricular in location.
View Answer
6b Answer A. Lateral ventricle attached to the septum pellucidum or ventricular wall.
|
Imaging Findings: Axial images from a contrast-enhanced MRI demonstrate a FLAIR/T2-hyperintense septal-based intraventricular mass attached to the septum pellucidum with cystic foci (A and B), no intrinsic T1 hyperintensity on precontrast T1w sequence (C), and few foci of nodular enhancement on the medial edge (D). The lesion has a bubbly appearance because of the numerous small cysts and overall features most consistent with a central neurocytoma.
Discussion: Central neurocytomas are WHO grade II lesions that are commonly seen in young adults and adolescents within the intraventricular location, predominantly in the lateral ventricle attached to the wall or septum pellucidum. Rarely, extraventricular occurrences of neurocytoma have been reported; however, the term “central” neurocytoma is reserved for the former. Complete surgical excision is standard management with role of postsurgical radiation therapy in selected cases such as incomplete resection. Given the propensity of this lesion to attach to the septum pellucidum adjacent to the Monro foramen, these lesions are associated with obstructive hydrocephalus and typically need ventriculostomy catheter for extraventricular CSF drainage for decompression along with gross total resection. Although extremely rare, central neurocytomas can occur in the spinal cord as a primary intramedullary lesion.
On Imaging, these are lobulated masses with frequent cystic foci giving it a bubbly appearance. About half of these lesions may also have calcifications. These are isointense to gray matter on T1w and hyperintense on T2w sequences. On postcontrast T1w, these lesions can show moderate to intense heterogenous enhancement. Periventicular T2 hyperintensity and prominent flow voids may also be present.
Reference: Chen CL, Shen CC, Wang J, et al. Central neurocytoma: a clinical, radiological and pathological study of nine cases. Clin Neurol Neurosurg 2008;110(2):129-136.
7 A 10-year-old male with abnormal head CT performed for progressive difficulty in swallowing, facial weakness, and visual abnormalities. An MRI was performed for further assessment; key images are shown below:
|
7a Based on the above images, what is the most likely etiology for the above changes?
A. Primary CNS glial neoplasm
B. Metastatic disease
C. Osmotic demyelination
D. Neuro-Behcet disease
View Answer
7a Answer A. Primary CNS glial neoplasm. Sagittal FIESTA and axial T2w images show infiltrative ill-defined T2-hyperintense pontine mass with an expansile morphology and heterogeneous enhancement. This appearance is very typical for a brainstem glioma.
7b What specific metabolite abnormality is depicted on the proton MRS?
A. Elevated choline and reduction in NAA
B. Elevated lactate
C. Reduced choline and elevated NAA
D. No abnormality is depicted with physiologic metabolite pattern.
View Answer
7b Answer A. Elevated choline and reduction in NAA. The findings on MR spectroscopy show elevated choline and reduced NAA. Typically, on physiologic spectra the choline peak is less than NAA peak; here, as we note, the pattern is reversed.
|
Imaging Findings: Sagittal high-resolution FIESTA image (A) shows a partially exophytic expansile lesion centered in the pons; the lesion is hyperintense on T2w sequence (C) and enhances on postcontrast T1w sequence (B). Single-voxel MR spectroscopy with spectra obtained from the lesion itself shows abnormal elevation of choline and reduced N-acetylaspartate. A definite lactate peak is not visualized.
Discussion: Brainstem gliomas (BSG) are a heterogeneous group of tumors with distinct subtypes with variable prognosis. They account for 10% to 20% of all intracranial tumors in children. They can uncommonly be seen in adults (median age of 30 to 35 years). In adults, the behavior and prognosis are similar to those of supratentorial gliomas. The rest of the discussion here is focused on pediatric BSG.
They are broadly classified into diffuse infiltrating type (about 80% of BSG) and focal gliomas. The prognosis largely depends on the site of origin. Also, BSG that are associated with NF1 have better prognosis.
Midbrain gliomas (tectal or tegmental) are generally low grade (pilocytic/grade II astrocytoma or ganglioglioma) and tend to be indolent. The treatment is often just shunting and imaging surveillance. Tumor-specific treatment is pursued in the unlikely event of tumor growth.
Most pontine gliomas are infiltrating fibrillary astrocytomas with poor prognosis. Irrespective of histologic grading, these have an aggressive course with a mean survival of 4 to 15 months. They tend to show extension into the midbrain superiorly and medulla inferiorly and through the middle cerebellar peduncle into the cerebellar hemisphere.
Less commonly, focal tumors can arise in the pons, and they show a good prognosis. Medullary tumors commonly show lower grade and better prognosis and have a higher incidence of exophytic growth compared to pontine tumors.
On CT, one-third of pontine tumors are hypodense, one-third are isodense, and most of the remainder show mixed density. In case of hemorrhage, tumors can have high density. Smaller midbrain tumors may not be well seen on CT. On MRI, BSGs are hypointense on T1 and hyperintense on T2/FLAIR. Patchy, focal, or ring enhancements within pontine tumors are common (either at presentation or developing during the course of the disease) as is hemorrhage; these features suggest an aggressive tumor. But enhancement in focal tumors and medullary tumors do not necessarily mean bad prognosis. Expanded brainstem that obliterates the prepontine cistern, compresses the fourth ventricle, and envelops the basilar artery is a strong indicator for BSG. Obstructive hydrocephalus occurs commonly with midbrain and medullary tumors and less commonly with pontine tumors. In case of aqueductal pattern of hydrocephalus, MRI is the investigation of choice to evaluate for midbrain tumors as smaller tumors may not be evident on CT and the patient may carry a diagnosis of just “aqueductal stenosis” instead. Midbrain tumor is better demonstrated with thin slices through this region, preferably a 3D T2 or steady-state sequence in sagittal plane.
Differential Diagnosis:
Demyelinating disease: Clinical picture is usually distinct with often multiphasic episodes; often multifocal in involvement with supra- and infratentorial lesions.
Infection (abscess and cerebritis): Tuberculosis
Neuro-Behcet syndrome: Generally in young adult population
References: Atlas SW, ed. Magnetic resonance imaging of the brain and spine, 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008.
Gupta N, Banerjee A, Haas-Kogan D, eds. Pediatric CNS tumors, 2nd ed. Berlin, Germany: Springer, 2010.
8 CT and MRI were performed for further workup of left-sided trigeminal neuralgia. Key images are shown below:
|
8a Which of the provided signal features are characteristics for this abnormality?
A. Extra-axial, T2 shine through on DWI, low attenuation on CT.
B. Extra-axial, restricted diffusion, low attenuation on CT, incomplete suppression on FLAIR
C. Intra-axial, T2 shine through on DWI, low attenuation on CT.
D. Extra-axial, T2 shine through on DWI, complete suppression on FLAIR
View Answer
8a Answer B. Extra-axial, restricted diffusion, low attenuation on CT, incomplete suppression on FLAIR.
8b Which of the above characteristics help differentiate this lesion from an arachnoid cyst?
A. T2 shine through on DWI
B. Restricted diffusion on DWI
C. Low attenuation on CT
D. High attenuation on CT
View Answer
8b Answer B. Restricted diffusion on DWI. The low attenuation on CT will be seen with both epidermoid and arachnoid cysts.
|
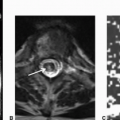
Imaging Findings: Axial DWI image showing restricted diffusion within an extra-axial mass in the left CP angle (A). This lesion has fluid intensity on CT and axial T2w sequence (B and D) with dilated appearance of the left Meckel cave and shows incomplete suppression on FLAIR (C), contrast the right Meckel cave CSF signal and signal within the globes on FLAIR to appreciate the incomplete fluid suppression within this lesion.
Discussion: Intracranial epidermoids are congenital tumor-like inclusion cysts and constitute about 1% of intracranial tumors. They are believed to be the result of sequestration of ectodermal element within the neural tube during the 3rd to 5th week of embryonic development. Acquired epidermoid is rare in the brain.
They are well-marginated lesions with irregular nodular surface and composed of a wall made up of stratified keratinized squamous epithelium surrounded by connective tissue. The cyst content is mainly derived from desquamated epithelial cells and includes debris, keratin, water, and cholesterol. These lesions do not contain dermal appendages.
The clinical presentation depends on the location and the degree of mass effect on adjacent neural structures. There may also be chemical meningitis secondary to leakage of cyst contents into subarachnoid space. The common locations are CP angle cistern (40% to 50% of lesions), fourth ventricle, sellar region, and less commonly intraparenchymal. Ten percent of tumors are extradural.
On imaging, they are thinly marginated, but irregular lesions, insinuating within the CSF space encasing and surrounding adjacent vessels and nerves. Calcification can be seen in 10% to 25% of cases. Rarely, intralesional hemorrhage can be present. On CT, the lesions are well circumscribed and hypodense and often show near CSF density, similar in appearance to arachnoid cyst. MRI is the investigation of choice. On T1 and T2 images, they look somewhat similar to an arachnoid cyst showing near CSF intensity or slight hyperintensity relative to CSF. On FLAIR images, these show incomplete signal suppression and can show some degree of heterogeneity. Diffusion images show restriction, which is the most useful MR imaging feature for diagnosis. Most of the lesions show no contrast enhancement; about a quarter show minimal rim enhancement.
Epidermoid can be hyperdense on CT because of hemorrhage or proteinaceous content (white epidermoid). These lesions can be hyperintense on T1 images and show variable signal on T2 images. Diffusion restriction is typically present. Malignant degeneration of epidermoid is extremely rare, but reported.
Differential Diagnosis:
Arachnoid cyst: especially on CT. On MRI, the differentiation is easy as epidermoids show incomplete FLAIR suppression and diffusion restriction unlike arachnoid cysts.
References: Chen CY, Wong JS, Hsieh SC, et al. Intracranial epidermoid cyst with hemorrhage: MR imaging findings. AJNR Am J Neuroradiol 2006;27(2):427-429.
Forghani R, Farb RI, Kiehl TR, et al. Fourth ventricle epidermoid tumor: radiologic, intraoperative, and pathologic findings. Radiographics 2007;27(5):1489-94. doi: 10.1148/rg.275075011.
9 A 55-year-old male, previously healthy, presents with altered mental status. Key images from CT and MRI are shown below:
|
9a Based on the above images what is the most likely diagnosis in an immunocompetent adult?
A. Primary CNS lymphoma
B. Subacute infarction
C. Cerebral abscess
D. Tumefactive demyelination
View Answer
9a Answer A. Primary CNS lymphoma. This lesion has classic characteristics of primary CNS lymphoma.
|
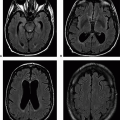
Image Findings: There is a right basal ganglia/thalamic mass, which is hyperattenuating on CT (A), is heterogeneously hypointense on T2w (B), and shows homogenous intense enhancement on postcontrast T1w sequence (C and D) and restricted diffusion (E and F). These features are typical for primary CNS lymphoma.
Discussion: Primary CNS lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma that affects the brain and is rarely isolated to the spinal cord, meninges, and orbit. It is usually of the large B-cell variety, which represents 5% to 7% of all primary brain tumors. By definition, extra-CNS disease is absent at the time of diagnosis.
Cerebral hemispheres are the commonest site with predilection of periventricular region and corpus callosum, followed by basal ganglia, thalami, and rarely posterior fossa. Lymphoma is a differential consideration for “butterfly” tumor. Leptomeningeal involvement occurs in about 12% tumors; dural involvement is rare. They tend to show angiocentric growth pattern. Most of the tumors tend to abut the CSF surface (either ventricular margin or the pia) with a tendency for subependymal spread. The mass effect is less pronounced for their size.
Over half of the tumors are solitary. The internal imaging characteristics depend on the immune status.
In immunocompetent patients, the tumors occur in older age group with a mean age of about 60 years. The most common presentation is solid uniformly enhancing lesion showing high cellularity and prominent perilesional edema. The high cellularity results in hyperdensity of CT, iso- to only mild hypointensity on T1-weighted images, intermediate to low signal on T2-weighted images, and restricted diffusion. Untreated lesions do not show calcification. Less commonly, they can be infiltrative and rarely can show necrosis, hemorrhage, and even no enhancement.
In immunocompromised patients, the lesions tend to occur at an earlier age (mean age of about 40 years). They show a higher incidence of necrosis, hemorrhage, and inhomogeneous enhancement.
Secondary lymphomas tend to involve dura and leptomeninges and only rarely present as isolated parenchymal mass.
PCNSL show increase in CBV on perfusion studies, but less than what is seen with glioblastomas. Reduction in ADC is more dramatic than what can be seen with tumefactive demyelination. Spectroscopy shows “tumor signature.” They are FDG avid.
Differential Diagnosis:
Glioblastoma: hemorrhage and necrosis are the hallmark, which are rare in PCNSL of immunocompetent patients. Abutment of CSF surface is more common with lymphoma. Uniform restricted diffusion is rare with glioblastoma.
Metastases: History and multiplicity are helpful. Diffusion restriction is less common with metastatic disease. They tend to be located at gray-white junction as opposed to near the CSF surface with lymphoma. Mass effect is disproportionately less for lesion site with lymphomas, as opposed to metastasis.
Tumefactive demyelination: Enhancement is more commonly peripheral, incomplete ring shaped, or comma shaped. Concentric rings of differing signal on T2-weighted images favor demyelination. Diffusion restriction can occasionally be seen with acute demyelination, but tends to be more pronounced with lymphoma. In difficult cases, follow-up is the key.
Toxoplasmosis: This is a differential consideration in the immunocompromised. Eccentric target sign favors toxoplasmosis. As opposed to lymphoma, toxoplasmosis is non-avid on PET and on thallium scan.
References: Lu SS, Kim SJ, Kim N, et al. Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol 2015;204(4):827-834. doi: 10.2214/AJR.14.12677.
Naidich TP, ed. Imaging of the brain. Expert Radiology Series. Philadelphia, PA: Saunders/Elsevier, 2013.
Tsiouris AJ, Sanelli PC, Comunale J. Case-based brain imaging, 2nd ed. New York, NY: Thieme, 2013.
10 A 35-year-old male with long-standing medically intractable seizures presents for initial evaluation with an abnormal MRI. No other comorbidities.
|
10a What is the best next step?
A. Neurosurgical consultation
B. Antiviral therapy
C. No further intervention is needed.
D. Routine annual follow-up can be performed.
View Answer
10a Answer A. Neurosurgical consultation. Given this is a chronic refractory epilepsy that has an identifiable structural substrate on imaging, a neurosurgical consultation with further relevant workup may be considered. In this case, this lesion is likely to represent dysembryoplastic neuroepithelial tumor.
10b Which of the following is true about the above entity?
A. Temporal lobe intracortical location is common for this lesion (DNET).
B. This is an aggressive malignant process.
C. White matter location is common.
D. This is a lesion typically seen in older <70 age group.
View Answer
10b Answer A. Temporal lobe intracortical location is common for this lesion (DNET). The other choices are untrue for this lesion, because it is commonly cortical, commonly in younger age group, and typically has a relatively benign clinical course.
Image Findings: Figure 1. There is left medial temporal lobe mass that expands the cortex with heterogeneous FLAIR hyperintensity (A), T2 hyperintensity (B), and lack of enhancement on postcontrast sequences (C). This appearance is most consistent with DNET. Ganglioglioma is a differential possibility; however, contrast enhancement is more commonly seen with the latter.
Figure 2. Companion case. There is a T2-hyperintense partly cystic lesion with a “bubbly” morphology in the right temporoparietal region (A); note that the bubbly foci do not suppress on FLAIR (B). There is heterogeneous enhancement (C).
Discussion: Dysembryoplastic neuroepithelial tumor (DNET) is a benign grade I glial-neuronal neoplasm, commonly seen in the second and third decades of life with male preponderance and presenting with intractable seizures.
It is peripherally located in the cortex with gyral expansion. Underlying white matter is often involved. Temporal lobe is the most common location followed by frontal lobe. Deeper location is uncommon and includes the basal ganglia, thalamus, pons, and cerebellum. Clinicoradiologic criteria for diagnosis include seizure onset before 20 years of age, absence of neurologic deficit, cortical location of the lesion and absence of mass effect/peritumoral edema. Typical radiologic features include a cortical-based wedge-shaped well-demarcated mass pointing toward the ventricle, presence of internal septations, absence of contrast enhancement, and scalloping of the overlying bone. On CT, these lesions are hypo- to isodense. On MRI, these lesions are T2 hyperintense, are generally T1 hypointense, and show “bubbly” appearance. Up to a third of lesions can show enhancement, generally nodular in morphology. Thin hyperintense rim on FLAIR images (seen in nearly three-fourths of cases) and high ADC values are other useful imaging features. Cystic changes are occasionally seen. Calcification can be seen but is rare. Associated cortical dysplasia is common.
These tumors generally do not show growth. Surgical resection is for seizure control and is curative. The histologic picture can occasionally be confusing; it is important to suggest the diagnosis based on preoperative imaging.
Differential Diagnosis:
Ganglioglioma: Cystic lesion with enhancing nodule is typical. Unlike DNET, calcification is common. Contrast enhancement is also more common in ganglioglioma.
Cortical dysplasia: Relatively non-mass-like appearance, cystic foci are unusual.
Low-grade astrocytoma: Tend to be centered in the white matter. Overlying bony change is unusual. May appear ill defined.
References: Fernandez C, Girard N, Paz Paredes A, et al. The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol 2003;24(5):829-834.
Gupta N, Banerjee A, Haas-Kogan D, eds. Pediatric CNS tumors, 2nd ed. Berlin, Germany: Springer, 2010.
Koeller KK, Henry JM. From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 2001;21(6):1533-1556. doi: 10.1148/radiographics.21.6.g01nv051533.
Raz E, Kapilamoorthy TR, Gupta AK, et al. Case 186: dysembrioplastic neuroepithelial tumor. Radiology 2012;265(1):317-320. doi: 10.1148/radiol.12100118.
11 A 13-year-old male who presents with constant suboccipital headache, blurring of vision, and truncal ataxia on examination. An MRI with contrast was performed; the images are provided below:
|
11a Based on these views, the abnormality showed above is centered in which of the following structures in the posterior fossa?
A. Midline cerebellar vermis
B. Brainstem
C. Lateral cerebellar hemispheres
D. Tentorium
11b In this age group and location, which is the most common malignant neoplasm?
A. Medulloblastoma
B. Juvenile pilocytic astrocytoma
C. Metastasis
D. Hemangioblastoma
View Answer




11b Answer A. Medulloblastoma. The most common malignant neoplasm in children is medulloblastoma, and it most commonly occurs in the posterior fossa.
|
Imaging Findings: There is a large mass centered in the fourth ventricle with restricted diffusion (A) and small cystic foci on T2w (B) and obstructive hydrocephalus (C). It is relatively hypointense on sagittal precontrast T1 (C) and shows intense heterogeneous enhancement on axial postcontrast T1w image (D). This appearance in the current age group is most consistent with a medulloblastoma.
Discussion: Medulloblastoma is the most common malignant pediatric brain tumor, but may also occur in adults. It is considered a primitive neuroectodermal tumor of the posterior fossa. Together with pilocytic astrocytoma, it forms the bulk of posterior fossa tumors in children. A majority of the tumors are seen in the first decade, the peak being 5 to 7 years of age. A small percentage of these tumors is seen with genetic tumor syndromes (Gorlin syndrome, Turcot syndrome, Li-Fraumeni syndrome, ataxia telangiectasia, Coffin-Siris syndrome).
Several histologic subtypes of medulloblastoma have been identified, the most common type being classic medulloblastoma. The other subtypes include desmoplastic/nodular medulloblastoma, medulloblastoma with extensive nodularity, large cell medulloblastoma, anaplastic medulloblastoma, and much rarer variants of medullomyoblastoma and melanotic medulloblastoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree