The brain may be affected by a variety of abnormalities in association with HIV infection. Knowledge of these abnormalities and their characteristic imaging features is important to neuroradiologists for the detection, diagnosis, and initiation of appropriate treatment. This review attempts to describe the imaging findings associated with brain disorders in HIV-seropositive patients and the rationales for integrating neuroradiologic techniques.
As the HIVAIDS epidemic approaches the 20th anniversary of the first mysterious reports of people with the syndrome, researchers and clinicians continue to grapple with the complexities of the virus. An estimated 36 million people worldwide are currently living with HIV, and some 20 million people have already died. If the number of new infections continues at the current rate, even the most devastating impact that can be anticipated from current levels of infection may seem minor compared with that of the future.
HIV enters the central nervous system (CNS) early in the course of infection, and the virus resides primarily at the cellular level in microglia and macrophages. Cerebrospinal fluid (CSF) analysis has demonstrated that HIV can enter the CNS soon after exposure, even before antibodies are detectable in the blood. HIV has been detected in the brain as early as 15 days after accidental intravenous inoculation . More than a decade ago, it became clear that the neuron dysfunction or death that underlies the clinical symptoms of HIV CNS disease could not result from direct infections of neurons. The mechanism of HIV-related brain injury remains poorly understood, however. The predominant pathogenesis is believed to involve the combined influence of HIV infection and the activation of immune-competent cells and their subsequent release of toxins, which leads to neuronal and astrocytic dysfunction .
The brain may be affected by a variety of abnormalities in association with HIV infection. Knowledge of these abnormalities and their characteristic imaging features is important to neuroradiologists for the detection, diagnosis, and initiation of appropriate treatment. Nowadays, patients who have AIDS form a considerable part of routine neuroradiologic work. The imaging modalities commonly used in patients who have HIV or AIDS are CT and MR imaging. In addition, nuclear medicine techniques, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET), are helpful. The spectrum of CNS abnormalities can be divided into three main categories: (1) HIV-associated lesions, (2) opportunistic infections, and (3) neoplasms.
This review attempts to describe the imaging findings associated with brain disorders in HIV-seropositive patients and the rationales for integrating neuroradiologic techniques.
HIV-associated central nervous system abnormalities
Like all viruses, HIV is a parasite that replicates within the living cells of the host. HIV infection of the CNS produces a range of cognitive, motor, and behavioral abnormalities . The syndrome observed after infection with HIV has been designated as HIV-associated dementia (HAD) . The prevalence of HAD was estimated in the early 1990s to be as high as 20% to 30% in those patients who have advanced HIV disease and low CD4 cell counts. HAD is now probably the most common cause of dementia worldwide among people aged 40 years or younger. With the advent of potent combination antiretroviral therapy (highly active antiretroviral therapy [HAART]), the incidence of HAD has decreased to as low as 10.5% . Although the incidence of HAD seems to be declining, the prevalence of milder yet debilitating neuropsychologic impairments may increase as individuals infected with HIV live longer. These findings support the hypothesis that HAART does not provide complete protection from the development of HAD.
The histopathologic marker of the HIV-infected brain is the presence of multinucleated giant cells (MGCs). Two neuropathologic correlates of cerebral infection by HIV include multinucleated giant cell encephalitis (MGCE) and progressive diffuse leukoencephalopathy (PDL) . MGCE and PDL may be end points, with a morphologic spectrum of HIV-induced changes in between.
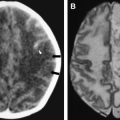
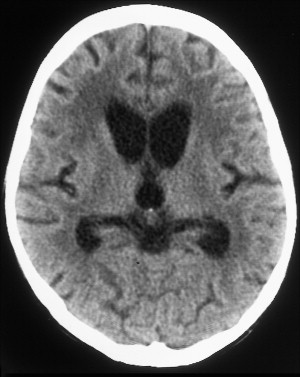
The most common reported imaging finding is cerebral atrophy ( Fig. 1 ). The predominant pattern of atrophy may be central (ventricular dilatation), peripheral (sulcal dilatation), or mixed (central and peripheral) . Levy and colleagues reported in their early CT study of 200 patients who had AIDS that 37.5% of the patients presented with cerebral atrophy. Serial CT studies have demonstrated progression of the atrophy over time in patients who have HAD. Jacobsen and colleagues reported cortical atrophy in one third of 400 patients who had AIDS with neurologic manifestations. Studies with symptomatic patients showed cortical atrophy in 85% of patients, which suggests that cortical atrophy may be relatively specific to patients who have neuropsychologic impairment. Aylward and colleagues found an association between HAD and specific gray matter volume reduction in the basal ganglia and posterior cortex and with generalized volume reduction of white matter. The total CSF volumes were significantly greater for HIV-positive patients who had HAD than for HIV-positive patients who did not have dementia.
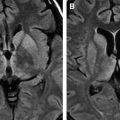
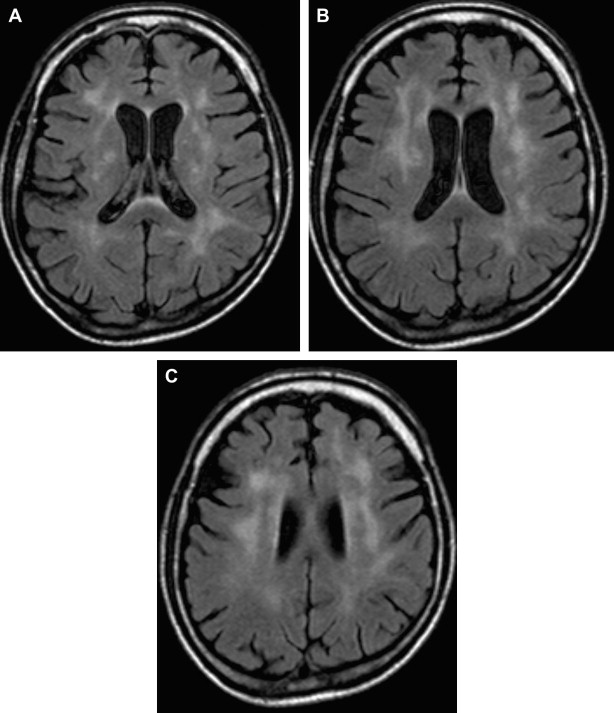
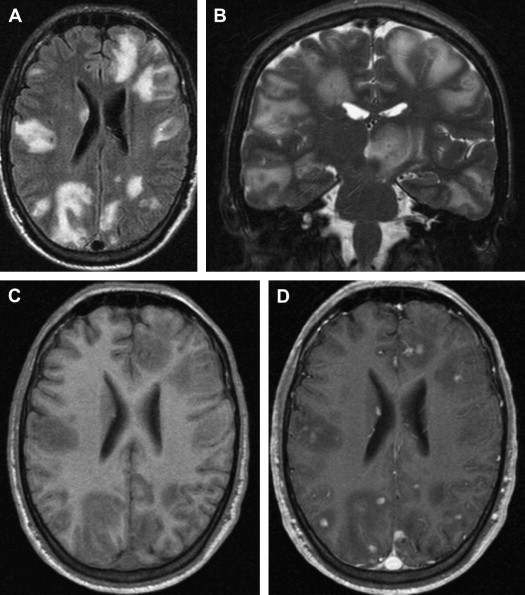
White matter lesions are the second most common MR imaging finding in patients who have HAD ( Fig. 2 ). The average frequency of white matter lesions in patients who have AIDS is 78%, with a range of 43% to 100% . A review of the MR imaging scans of 365 patients who had AIDS revealed signal abnormalities in the white matter in 31% of patients , with four patterns observed: diffuse, patchy, focal, and punctate. The white matter lesions demonstrate high signal on T2-weighted MR imaging sequences and usually are isointense or minimally hypointense on T1-weighted MR imaging sequences. Enhancement and mass effect are not present. Two distinct MR imaging patterns on T2-weighted imaging were observed, which allows a distinction between the two types of HAD: (1) diffuse, bilateral, and symmetric high signal intensity involvement of the white matter (butterfly-like; see Fig. 1 ) and (2) bilateral, scattering, high signal intensity lesions in the white and gray matter (patchy, see Fig. 2 ) . The appearance of MR imaging scans most likely reflects an increase in water content and the leakage of serum proteins resulting from altered vascular permeability, possibly related to the presence of circulating cytokines. Prospective studies with long-term follow-up comparing asymptomatic with symptomatic patients have shown that the percentage of white matter abnormalities significantly increases with disease progression .
Although MR imaging is the most sensitive imaging modality for depicting the effects of HIV in the brain, it is nevertheless not sensitive enough to show early pathologic involvement. Several investigators have claimed that neuroimaging studies are relatively insensitive in the detection of early changes in the brain attributable to HIV infection. Compared with the postmortem studies, MR imaging underestimated the presence of HIV-related lesions in the brain . Fluid-attenuated inversion recovery (FLAIR) techniques have been shown to improve conspicuity of the lesions in the periventricular and subcortical locations significantly .
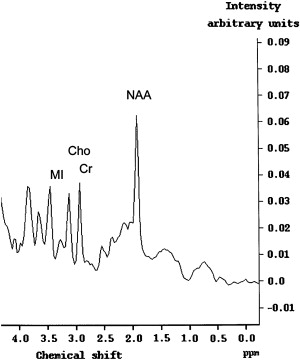
The data from recent studies suggest that proton ( 1 H) magnetic resonance spectroscopy (MRS) could potentially be more sensitive in detecting early CNS involvement by HIV than MR imaging . Results of recent studies have shown that the metabolite pattern in patients who have HAD indicates decreased N-acetylaspartate (NAA) and elevated choline (Cho) and myoinositol (MI) levels that are not observed in healthy subjects ( Fig. 3 ) (lower NAA/creatine [Cr] ratio, increased Cho/Cr ratio, and increased MI/Cr ratio) . The findings indicate that diffuse brain involvement in AIDS can be marked not only by a loss of neurons, as indicated by increased NAA levels, but by increased MI levels. Chang and colleagues recently reported an increased Cho/Cr ratio in the frontal white matter and in the basal ganglia and an elevated MI/Cr ratio and MI concentrations in the basal ganglia.
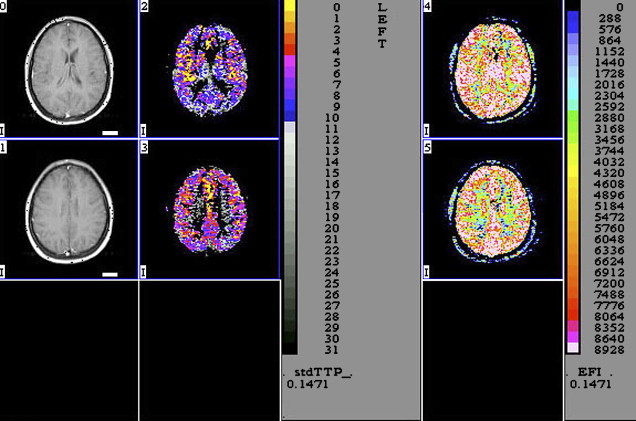
Perfusion MR imaging can also detect abnormalities that correlate with disease severity in HAD ( Fig. 4 ). Chang and colleagues evaluated the regional cerebral blood flow (rCBF) in 19 patients who had early stages of HAD and correlated the results with 15 healthy seronegative subjects. The patients who had HAD had a statistically significant decrease of rCBF bilaterally in the inferior lateral frontal cortices and an increase in the posterior inferior parietal white matter. Because pMR imaging is faster and safer than nuclear medicine techniques, these investigators suggested that pMR imaging can be used to monitor rCBF changes in patients who have HIV initially and during therapy.
Diffusion tensor imaging (DTI) is another promising MR imaging technique with the possibility to measure tissue anisotropy, thus providing details on tissue microstructure . Calculating the diffusion constant and anisotropy in the subcortical white matter and corpus callosum in patients who had HIV, Filippi and colleagues recently found abnormalities despite normal-appearing white matter on conventional MR imaging. Patients who had advanced HIV disease (high viral load) had the highest diffusion constant elevations and largest anisotropy decreases. The diffusion changes observed in the study of Filippi and colleagues may be a result of activation of inflammatory pathways in HIV infection of the brain. In another study with 60 HIV-positive patients, a statistically significant decrease of fractional anisotrpy (FA) was found in the genu of the corpus callosum in patients who had HIV compared with controls. FA was reduced in the frontal white matter and hippocampi in patients who had HIV compared with controls. Differences were not statistically significant, however . In addition, DTI did not correlate well with virologic and immunologic parameters in that particular study. It seems that FA and apparent diffusion coefficient (ADC) measurements are not sufficient in identifying patients who have early HIV infection.
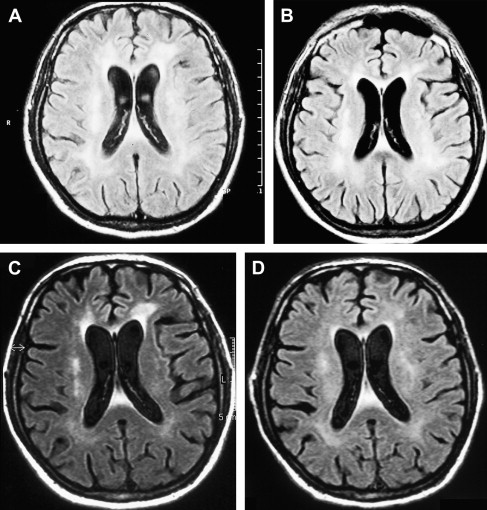
An increased knowledge of the pathogenesis of HIV, the development of sensitive measurements for such viral parameters as viral load determinations, and the availability of new classes of antiretroviral drugs have profoundly improved the therapeutic management of HIV infection . The results of several recent studies suggest that MR imaging and MRS may help in the clinical management of patients who have HAD and are receiving potent antiretroviral therapy and can be used to characterize changes attributable to HAART. A combination of antiretroviral drugs in patients who have HAD may result in stabilization or even regression of white matter signal intensity abnormalities observed on MR imaging ( Fig. 5 ) . The progression of white matter lesions on initial follow-up studies should not be mistaken for therapy failure, because the progression of MR imaging findings is likely the result of postinflammatory reactions attributable to immune reconstitutive effects after the initiation of HAART . Some other observations could also be important in understanding the discrepancies between the evolution of MR imaging findings and cognitive function in patients who have HAD and are receiving HAART. The progression of cerebral atrophy was observed in all patients who had HAD and underwent HAART in one study, suggesting that despite potent therapy, the neuronal damage seems to progress without clinical manifestations .
Opportunistic infections
Viral infections
Cytomegalovirus
Cytomegalovirus (CMV) is a member of the herpesvirus family; in adults, the infection is a result of the reactivation of a latent infection, most commonly presenting as mild infection mimicking mononucleosis. In immunocompromised patients, CMV can produce a variety of clinical syndromes. CMV infections have been described with increasing frequency in patients who have AIDS . Five distinct neurologic syndromes attributable to the CMV infection have been described: retinitis, myelitis or polyradiculopathy, diffuse micronodular encephalitis, ventriculoencephalitis, and mononeuritis multiplex.
Diffuse micronodular encephalitis resembles HIV encephalitis histologically. Small microglial nodules and inclusion-bearing cytomegalic cells are widely distributed in the cortex, basal ganglia, brain stem, and cerebellum. In an autopsy series of 30 cases of CMV encephalitis, 76% of the patients had microglial nodules containing inclusion-bearing cells and 24% had CMV inclusions outside the nodules . A distinct clinical syndrome of dementia associated with CMV has been described and compared with HAD . Those two dementia syndromes differ in clinical presentation, course, associated electrolyte disturbances, and imaging findings. The most common imaging findings in patients who have CMV encephalitis are cortical atrophy, periventricular enhancement, and diffuse white matter abnormalities . Generalized atrophy is the most commonly reported CT abnormality, but it is a nonspecific finding seen frequently in patients who have AIDS . Periventricular enhancement is also not diagnostic, but it has been described in cases of lymphoma, toxoplasmosis, and other infections. White matter disease occurs as a result of inflammation of the subependymal region and spread of the infection to the adjacent astrocytes of the white matter with infectious demyelination . In six patients who had pathologically confirmed CMV infection of the CNS, Hassine and colleagues found atrophy in three cases, subependymal nodular lesions without enhancement in two cases, and ventriculitis in one case.
Patients who have ventriculoencephalitis have a more acute onset, with death usually occurring quickly. Autopsy reveals periventriculitis, ependymal and subependymal necrosis, and associated inclusion-bearing CMV cells .
Rarely, cerebral mass lesions attributable to CMV could be observed in patients who had AIDS. In one study, two cases of cerebral mass lesions attributable to CMV were described . In both cases, a large contrast-enhancing mass was seen in the frontal lobe with surrounding edema. Both patients described in the study died despite treatment. CMV should be considered in the differential diagnosis of mass lesions in patients who have AIDS with severe immune deficiency.
CMV infection can also be present as choroid plexitis. In one reported case, a contrast-enhanced CT scan showed marked enhancement of the slightly enlarged right plexus. MR imaging confirmed the findings and the absence of enhancement of ependyma . Infections of the choroid plexus are not common; nevertheless, CMV should be included in the causes of choroid plexitis in patients who have AIDS.
Infection of the CNS is difficult to diagnose while the patient is alive because CMV is difficult to culture from CSF. The recent development of the polymerase chain reaction technique has allowed isolation of CMV based on the presence of DNA within the CSF . The discrimination between HIV and CMV-associated CNS disease is often difficult using clinical and imaging findings. MRS could be potentially useful in such cases. In one study, MRS was used to distinguish HIV encephalitis from CMV encephalitis . These findings suggest that a larger Cho signal and a smaller NAA signal could be inferred within the white matter abnormalities attributable to HIV encephalitis or encephalopathy compared with CMV encephalitis.
Bacterial infections
Mycobacteria
The increased incidence of tuberculosis in developed countries is clearly related to the increase in AIDS. At present, approximately 30% of patients who have tuberculosis are HIV-positive; conversely, 5% to 9% of patients who have AIDS develop tuberculosis . Currently, AIDS is considered the main risk factor for the development of tuberculosis . It is known that clinical manifestations, management, and epidemiology are altered in patients who have AIDS . HIV-positive patients may develop active tuberculosis by two different mechanisms: reactivation of a latent infection or rapid progression of a newly acquired infection . Another important fact is that tuberculosis may appear in the early stages of immunodeficiency. The most striking clinical feature is the extremely high frequency of extrapulmonary involvement.
Approximately 10% of all patients who have AIDS with tuberculosis present with CNS disease .
Basically, tuberculous infection of the CNS results in tuberculous meningitis, which is characterized by a thick gelatinous exudate with a predilection for the meninges covering the base of the brain. Meningeal enhancement on enhanced CT and MR imaging in the basal cisterns and over the convexity of the brain could be seen in approximately 36% to 61% of the cases ( Fig. 6 ) .
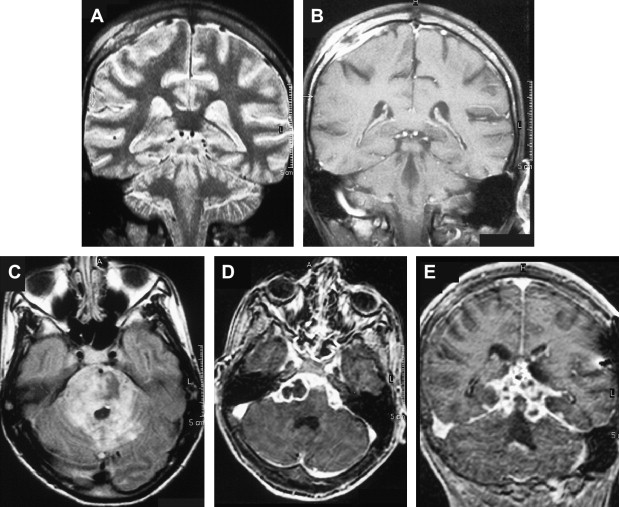
Involvement of the vessels that course through the subarachnoid space by the tuberculous inflammation results in narrowing, and occlusions of the vessels and subsequent infarctions, which are usually located in the middle cerebral artery territory and small perforating arteries supplying the basal ganglia . Diffusion-weighted MR imaging is helpful in detecting early infarcts, and magnetic resonance angiography (MRA) has been reported to be useful in showing the tuberculosis-induced vascular pathologic changes . Communicating-type hydrocephalus is the most common complication in meningeal tuberculosis .
Villoria and colleagues reported hydrocephalus in 51% of patients in their series of 35 patients who had AIDS-related CNS tuberculosis.
Parenchymal forms of the tuberculous infection include tuberculomas, tuberculous abscesses, and focal tuberculous cerebritis. In the AIDS population, parenchymal involvement is not often seen. Tuberculous granulomas (tuberculomas) result from hematogenous spread of infection or from extension of meningitis into the parenchyma. Tuberculomas may be solitary or multiple and can be located anywhere in the brain but are predominantly found in the supratentorial compartment . The radiologic features of tuberculomas depend on the maturity of the lesion. On CT, mature granulomas are ring-enhancing lesions. The “target sign” was first described as a pathognomonic feature for CNS tuberculoma , representing central calcification or punctate enhancement surrounded by a zone of hypodensity and a rim of enhancement. Later reports have shown that the target sign on CT could also be caused by other lesions, such as toxoplasmosis, lymphoma, or brain abscess . The tuberculomas appear isointense with a hyperintense ring on T1-weighted MR imaging and have a variable signal on long echo time (TE) images. Some tuberculomas are hypointense on T2-weighted images. The possible explanation for the low signal of tuberculomas on T2-weighted images is the presence of paramagnetic free radicals produced by macrophage activity. Other tuberculomas have high signal on long TE images because of central liquefactive necrosis . Kim and colleagues compared MR imaging findings with pathologic features in tuberculomas and found that the outer enhancing portion consisted of a layer of collagenous fibers and that inflammatory infiltrates showed on MR imaging as a hypointense ring on T2-weighted images; central caseation necrosis was recognized as isointense or hypointense on all pulse sequences. Healed tuberculomas do not enhance but may calcify.
Tuberculous abscess is a true pyogenic lesion that demonstrates a typical imaging appearance of pyogenic abscesses: hyperintense on T2-weighted MR imaging and hypointense on T1-weighted MR imaging. Typically, tuberculous abscesses are multiloculated, are larger than tuberculomas, and show ring-like enhancement . Differentiation of tuberculous abscess from pyogenic abscess is important for patient management. A recent study has shown that with MRS combined with MR imaging, it might be possible to distinguish those two entities . In that study, all pyogenic brain abscesses had lipid and lactate peaks and amino acid peaks. Patients who had tuberculous abscesses had only lipid and lactate levels. The magnetization transfer (MT) ratio from the wall of the pyogenic abscess was significantly higher than that from the tuberculous abscess wall. In another series of 28 tuberculomas, lipid peaks were seen in 86% of the tuberculomas . Large resonances of fatty acids at 1.3 ppm and 0.9 ppm, which are assigned to a methylene group, and terminal methyl groups of fatty acids described in tuberculomas are attributable to the high lipid content of caseous material .
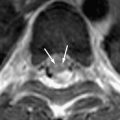
Focal tuberculous cerebritis is a rare form of tuberculosis characterized by intense gyral enhancement on CT scans . Calvarial tuberculosis is another rare manifestation of extrapulmonary tuberculosis. The presence of lytic lesions of the skull in a young individual in an endemic area should raise the suspicion of tuberculosis. Patankar and colleagues have described CT findings in five cases of tuberculosis of the calvarium. A case of proved calvarial tuberculosis in a young patient who had AIDS from the author’s institution is shown in Fig. 6 .
Fungal infections
Cryptococcosis
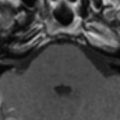
CNS infection caused by the saprophytic yeast-like fungus Cryptococcus neoformans is the most common form of fungal infection in patients who have AIDS. The infection is the result of a newly acquired infection with hematogenous dissemination of the infection from the lung to the CNS. Approximately 5% to 10% of patients who have AIDS develop CNS cryptococcosis. Cryptococcal meningitis is the most common manifestation, wherein the subarachnoid spaces are thickened and filled with multiple organisms and capsular material. CT scans rarely show meningeal enhancement, whereas enhanced T1-weighted MR imaging may demonstrate meningeal disease, but this is the exception rather than the rule . In a case of prominent meningeal enhancement on MR imaging, causes other than cryptococcosis should be considered first.
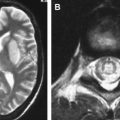
From the subarachnoid space, cryptococcus extends along the Virchow-Robin perivascular spaces into the basal ganglia, thalami, midbrain, and cerebellum. The Virchow-Robin spaces become dilated, without involvement of the brain parenchyma. On MR imaging, widened perivascular spaces are recognized as multiple, bilateral, and small round- or oval-shaped lesions usually located in the basal ganglia, which show high signal on T2-weighted MR imaging and have signal slightly higher than the CSF on T1-weighted MR imaging ( Figs. 7 and 8 ). The reason for the signal intensity characteristics is the mucoid content produced by fungi. Perivascular spaces are anatomically outside the brain, an inflammatory response is absent, there is no invasion of the brain parenchyma, and contrast enhancement is therefore always absent.
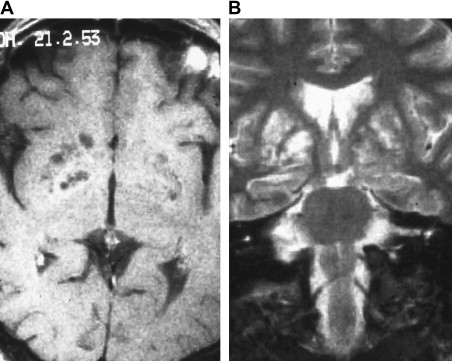
With disease progression, dilated perivascular spaces become confluent and cystic lesions develop called “gelatinous pseudocysts” or “soap bubbles” (see Fig. 8 ). Those lesions do not have a capsule and contain mucinous material and fungal organisms. Dentate nuclei are shown to be frequently involved . On MR imaging, the appearance does not differ from the dilated Virchow-Robin spaces, because mucinous material shortens the T1 relaxation time and the lesions appear isointense to the cortex. Enhancement and mass effect are also absent.
Cryptococcoma is the only parenchymal form of the cryptococcal CNS infection. The lesions result from the direct invasion of the brain by the fungus, with the development of a granulomatous reaction. On CT, cryptococcomas are hypodense with high signal on T2-weighted MR imaging and low signal on T1-weighted MR imaging. On enhanced imaging, the lesions usually demonstrate a ring-like or nodular enhancement and cannot be distinguished from granulomas of other origin. Recently, imaging findings with FLAIR and DWI of an intracerebral granuloma were described . On FLAIR images, cryptococcoma showed signal higher than that of the CSF and a mosaic pattern on diffusion-weighted images was observed, reflecting the inorganic structure of the lesion.
Aspergillosis
Aspergillus is a frequent pathogen in the CNS, accounting for 18% to 28% of all fungal brain abscesses, and is the most common CNS complication after bone marrow transplantation . Aspergillus fumigatus is the most common human pathogen. Because of the high mortality rate of 85% to 100% and difficulties in treating aspergillosis, it is important to raise suspicion early. Cerebral aspergillosis usually occurs after hematogenous dissemination from an extracerebral focus or is a result of contiguous spread of the infection from the paranasal sinuses. Meningitis, abscess or granuloma, vascular invasion with thrombosis and infarction, and hemorrhage and aneurysm formation are manifestations of cerebral aspergillosis .
Pathologically, hyphal elements invade cerebral vessels, resulting in thrombosis and infarctions. Sterile infarctions become septic when the fungus erodes the wall of the vessel with extension into the brain parenchyma, with inflammatory reactions and necrosis. MR imaging is the method of choice in detecting cerebral infarctions because of aspergillosis. In addition, a recent study has shown that diffusion imaging allows early diagnosis of CNS aspergillosis . DWI revealed multiple signal intensity abnormalities consistent with infarctions, whereas conventional MR imaging sequences were still normal . Intracerebral parenchymal lesions in aspergillosis have a central zone of hemorrhagic necrosis with sparse presence of fungi. On MR imaging, these lesions usually have low signal centrally or peripherally on T2-weighted images ( Fig. 9 ) . Cox and colleagues showed that low peripheral signal is attributable to accumulation of fungi containing iron, magnesium, and manganese in addition to blood breakdown products. Low signal on T2-weighted MR imaging is not specific for aspergillosis, and it may be seen in tuberculomas or cysticercosis, for example. In one series, 14 of 36 lesions showed areas of low signal intensity located centrally, and in 8 of 36 lesions, low signal was present peripherally . Contrast enhancement is usually not present, depending on the severity of the immunocompetence. Dietrich and colleagues have observed faint enhancement in 15 of 36 lesions in patients who had bone marrow transplantation and cerebral aspergillosis.
Parasitic infections
Toxoplasmosis
Cerebral toxoplasmosis results from infection by an intracellular protozoan, Toxoplasma gondii. In the United States, 20% to 70% of adults are seropositive . After the acute infection, the latent form, called encysted bradyzoites, remains in the tissues until a decline in immunity. Rupture of the cysts releases the free tachyzoite, which causes acute illness. Toxoplasma is found in 10% to 34% of all AIDS autopsies .
In patients who have AIDS, Toxoplasma causes necrotizing encephalitis. The lesions have three well-defined zones: an avascular necrotic center, an intermediate zone with intense inflammatory reaction, and a peripheral zone with an encysted form of Toxoplasma .
On nonenhanced CT scans, toxoplasma lesions are hypodense with edema and mass effect. Solid, nodular-enhancing, or ring-enhancing lesions are typically observed on postcontrast studies. The most common locations are the basal ganglia and the corticomedullary junction. The number of detectable lesions significantly increases with a double-dose delayed technique . Pathologic-radiologic correlation showed that the radiologic appearance correlates well with the pathologic findings; the ring-enhancing lesions correlate with an inflammatory zone, the hypodense center correlates with an avascular necrosis, and edema correlates with the peripheral zone .
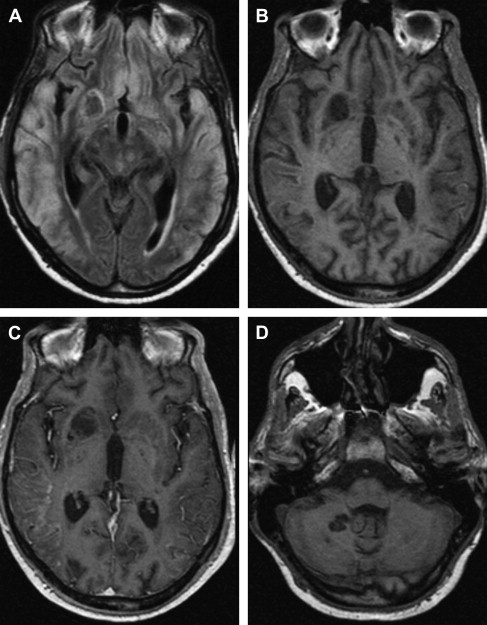
On T1-weighted MR imaging, toxoplasma lesions have isointense to low signal centrally. Signal intensity on T2-weighted images depends on the stage of the lesion, which could be isointense, hypointense, or hyperintense ( Fig. 10 ). Enhanced T1-weighted images reveal ring or nodular enhancement ( Fig. 11 ).
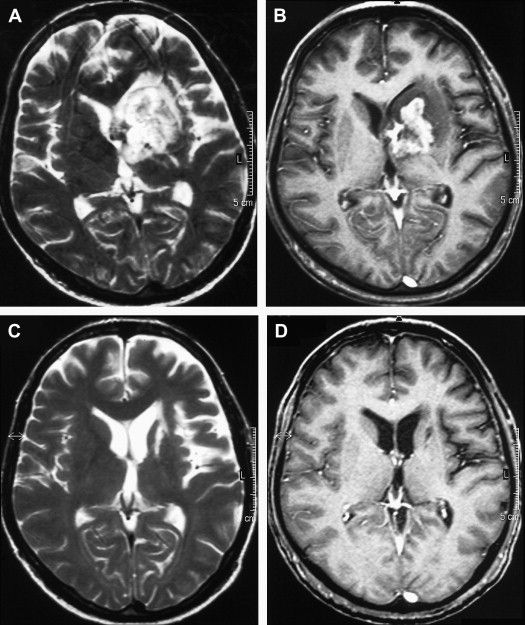
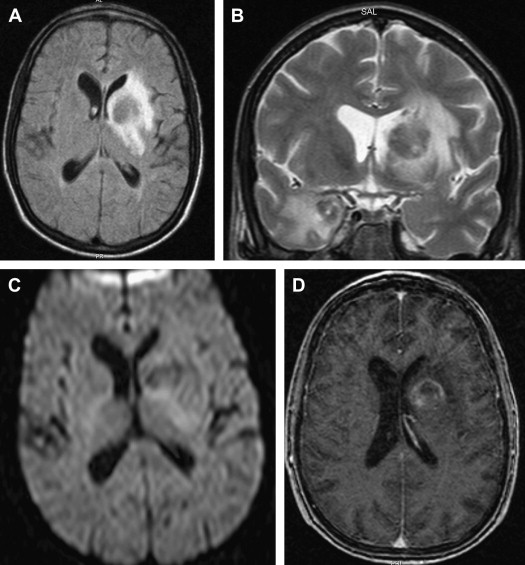
Approximately 10 days after the initiation of antitoxoplasma therapy, a decrease in the number and size of the lesions, with a reduction in edema and mass effect, should be observed on follow-up MR imaging examinations. Full resolution of the lesions may take 6 months , and healed foci may calcify or show changes consistent with leukomalacia (see Fig. 10 ). Lifelong maintenance of therapy is necessary, because discontinuation of treatment results in recurrence. Encysted forms of Toxoplasma cannot be treated with therapy; thus, toxoplasma lesions are never entirely eradicated.
Based on findings on conventional MR imaging sequences, cerebral toxoplasmosis cannot be distinguished from primary cerebral lymphoma. Early reports about MR imaging findings of toxoplasmosis and lymphoma in patients who had AIDS describe solitary lesions, which suggest lymphoma, and multiple lesions, which suggest toxoplasmosis . Further studies have shown that the number of the lesions, their signal intensity, their location, and their appearance on postcontrast images are not reliable factors in differentiation between those two entities. New MR imaging methods have been introduced to improve differentiation, such as MRS and pMR imaging.
The MRS pattern of toxoplasma lesions is nonspecific, consistent with anaerobic inflammation within the abscess ( Fig. 12 ). In lymphoma, an increase of lactate and lipids and an elevated Cho peak have been described. Chinn and colleagues have studied MR imaging spectra from 18 toxoplasma lesions and nine lymphomas at 1.5 T with a TE of 135 milliseconds. Visual analysis in their study failed to differentiate between Toxoplasma and lymphoma. An important variable for MR imaging spectra is the maturity of the lesions and the presence of necrosis. At some stage, all intracranial processes destroy brain parenchyma, and in patients who have AIDS, lymphomas are usually necrotic tumors. Therefore, necrotic lymphoma has compounds similar to necrotic toxoplasma abscess. The presence of lipids is seen in both entities as a result of brain destruction. A relatively immature toxoplasma lesion is more cellular, resulting in a spectrum with no lipid, increased Cho, and decreased NAA. It is crucial to place the voxel for spectroscopic analysis over the cellular portion of the lesion. In another series of 11 toxoplasma lesions and eight lymphomas of the brain in patients who had AIDS, MRS was considered a potentially specific noninvasive adjunctive method for differential diagnosis of focal brain lesions in AIDS . Toxoplasma was correctly diagnosed in 11 of 11 cases, and lymphoma was correctly diagnosed in eight of eight cases. The lactate peak was greatest in lymphoma in that study. Although much work has been done, MRS must be seen as a valuable adjunctive tool and cannot replace conventional MR imaging in differentiation of toxoplasmosis and lymphoma.
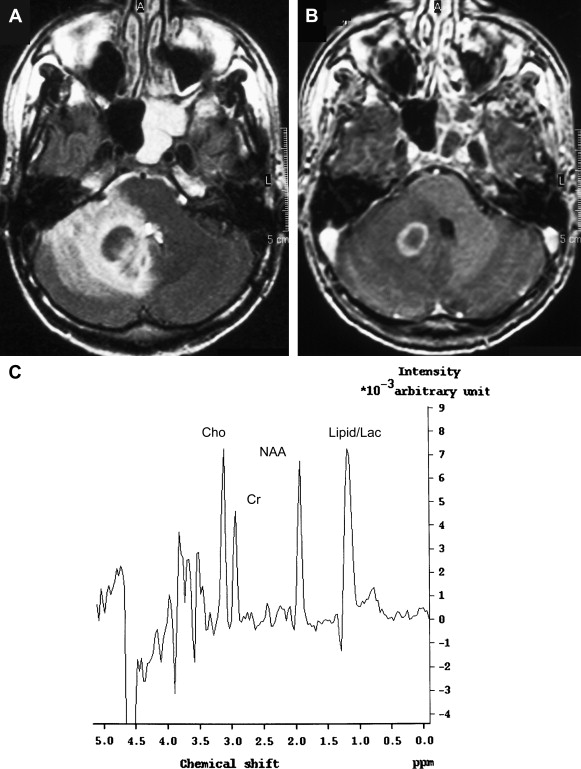
pMR imaging is another potential noninvasive method that may allow differentiation between toxoplasmosis and lymphoma in patients who have AIDS. Prospective evaluation of 13 patients who had focal brain lesions with pMR imaging showed reduced regional cerebral blood volume (rCBV) in toxoplasma lesions and increased rCBV in lymphoma . Reduced rCBV in toxoplasmosis is probably attributable to a lack of vasculature within the abscess, whereas the hypervascularity of lymphoma is the reason for increased rCBV in lymphoma.
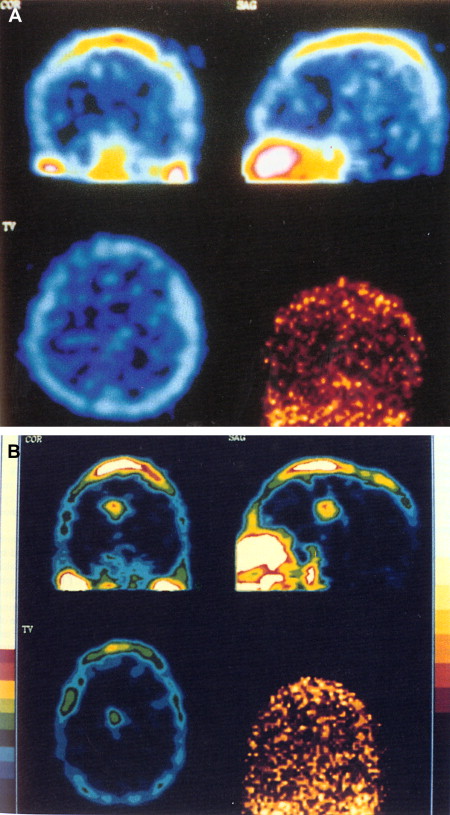
The use of thallium-201 ( 201 TI) brain SPECT in patients who have AIDS has been proved to be helpful in distinguishing toxoplasmosis from lymphoma . Thallium is a potassium analogue with uptake in active tissue, with a half-life of approximately 73 hours . After intravenous administration, 201 TI rapidly disappears from the blood and increased activity is usually seen in the orbits, the base of the skull, the scalp, and the nasopharyngeal region. Normally, there is no uptake of 201 TI in the brain. Positive 201 TI brain SPECT is suggestive of CNS lymphoma, and negative uptake suggests infection (toxoplasmosis) in patients who have AIDS ( Fig. 13 ) . A study of 37 patients who had AIDS and focal brain lesions evaluated with 201 TI SPECT has shown the benefit of 201 TI SPECT in the differentiation between lymphoma and infectious lesions. All lymphomas in this series showed uptake of 201 TI in contrast to infections, which showed negative uptake . Contrary to the results of Ruiz and colleagues , the results from a recent prospective study on 14 patients who had AIDS and focal brain lesions suggest the inability of 201 TI SPECT to differentiate lymphoma from toxoplasmosis . The accuracy was 57% in that study, with a positive predictive value of 43% and a negative predictive value of 71%. A combination of 201 TI SPECT and toxoplasma serology may improve diagnostic accuracy for toxoplasmosis . A combined approach with 201 TI SPECT and Epstein-Barr virus DNA polymerase chain reaction in CSF provides high diagnostic accuracy for cerebral lymphoma . Thallium and gallium scans were reviewed in 40 patients who had AIDS with focal brain lesions. All patients who had lymphomas and gliomas had positive thallium and gallium scans. Patients who had infections (toxoplasmosis and tuberculosis, mycobacterial infection, and cryptococcosis) had positive gallium scans and negative thallium scans. Negative thallium and gallium scans were also seen in patients who had infarcts.