The potential benefits of peptide-based immunotherapy for pediatric brain tumors are under investigation. Treatment-related heterogeneity has resulted in radiographic challenges, including pseudoprogression. Conventional MR imaging has limitations in assessment of different forms of treatment-related heterogeneity, particularly regarding distinguishing true tumor progression from efficacious treatment responses. Advanced neuroimaging techniques, including diffusion magnetic resonance (MR), perfusion MR, and MR spectroscopy, may add value in the assessment of treatment-related heterogeneity. Observations suggest that recent delineation of specific response criteria for immunotherapy of adult brain tumors is likely relevant to the pediatric population and further validation in multicenter pediatric brain tumor peptide-based vaccine studies is warranted.
Key points
- •
Peptide-based immunotherapy for pediatric brain tumors is associated with the presence of treatment-related heterogeneity, including that of pseudoprogression.
- •
Conventional MR imaging has limitations in the assessment of treatment-related heterogeneity, particularly regarding distinguishing true tumor progression from efficacious treatment responses.
- •
Advanced neuroimaging techniques, including diffusion magnetic resonance (MR), perfusion MR, and MR spectroscopy (MRS), may add value in the assessment of treatment-related heterogeneity.
- •
Recent delineation of specific response criteria for immunotherapy of adult brain tumors (Immunotherapy Response Assessment in Neuro-Oncology [iRANO]) is likely to be relevant to the pediatric population.
Introduction
There has been significant progress in the field of immunotherapy, within oncology, with recent Food and Drug Administration approval of immunotherapeutics for metastatic melanoma and non–small cell lung cancer and the advent of multiple immunotherapy clinical trials for primary and metastatic adult brain tumors. These adult immunotherapy studies have identified unique responses in regard to treatment response heterogeneity (as characterized by pseudoprogression, delayed responses, therapy-induced inflammation, and so forth) and resulting radiographic challenges. As such, new guidelines have been recently published by the iRANO group to allow for refinement of response assessment criteria for neuro-oncology patients receiving immunotherapy. These iRANO criteria suggest that among adult patients who demonstrate imaging findings meeting RANO (response assessment in neuro-oncology) criteria for progressive disease within 6 months of initiating immunotherapy, including the development of new lesions, confirmation of radiographic progression on follow-up imaging is recommended provided that the adult patient is not significantly worse clinically.
The authors’ institutions is currently engaged in multiple peptide-based vaccine trials for children with diffuse intrinsic pontine glioma (DIPG), recurrent high-grade glioma, recurrent low grade-glioma, and recurrent ependymoma, The authors have recently described the occurrence of heterogeneous treatment response (including pseudoprogression), which has remarkable similarity with what has been seen in some adult immunotherapy studies. The purpose of this review article is to highlight the authors’ initial experience with regard to the emerging radiographic challenges related to heterogeneous treatment response, including that of pseudoprogression, with the use of peptide-based vaccine therapy in pediatric brain tumors. The authors’ initial experience with some of the advanced neuroimaging techniques, including diffusion MR and MRS, is also described to help address some of these radiographic challenges.
Introduction
There has been significant progress in the field of immunotherapy, within oncology, with recent Food and Drug Administration approval of immunotherapeutics for metastatic melanoma and non–small cell lung cancer and the advent of multiple immunotherapy clinical trials for primary and metastatic adult brain tumors. These adult immunotherapy studies have identified unique responses in regard to treatment response heterogeneity (as characterized by pseudoprogression, delayed responses, therapy-induced inflammation, and so forth) and resulting radiographic challenges. As such, new guidelines have been recently published by the iRANO group to allow for refinement of response assessment criteria for neuro-oncology patients receiving immunotherapy. These iRANO criteria suggest that among adult patients who demonstrate imaging findings meeting RANO (response assessment in neuro-oncology) criteria for progressive disease within 6 months of initiating immunotherapy, including the development of new lesions, confirmation of radiographic progression on follow-up imaging is recommended provided that the adult patient is not significantly worse clinically.
The authors’ institutions is currently engaged in multiple peptide-based vaccine trials for children with diffuse intrinsic pontine glioma (DIPG), recurrent high-grade glioma, recurrent low grade-glioma, and recurrent ependymoma, The authors have recently described the occurrence of heterogeneous treatment response (including pseudoprogression), which has remarkable similarity with what has been seen in some adult immunotherapy studies. The purpose of this review article is to highlight the authors’ initial experience with regard to the emerging radiographic challenges related to heterogeneous treatment response, including that of pseudoprogression, with the use of peptide-based vaccine therapy in pediatric brain tumors. The authors’ initial experience with some of the advanced neuroimaging techniques, including diffusion MR and MRS, is also described to help address some of these radiographic challenges.
Conventional MR Imaging
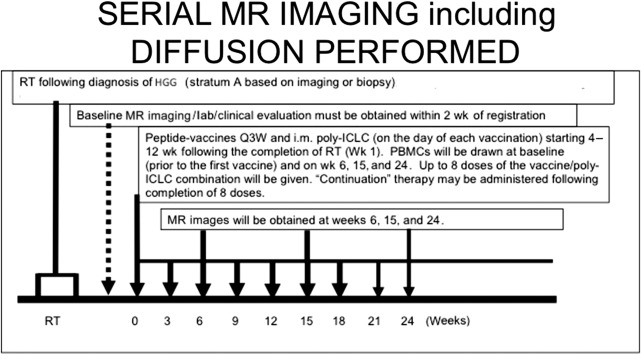
The authors have noted multiple forms of treatment-related heterogeneity in different pilot studies of peptide-based vaccine therapy for pediatric brain tumors, particularly in DIPG, recurrent supratentorial high-grade tumors, and recurrent low-grade gliomas. Conventional MR imaging supplemented with MRS, diffusion MR, and perfusion MR was typically performed serially at regular intervals depending on the specific protocol while on the peptide-based vaccine therapy ( Fig. 1 ) (ie, every 6 weeks for newly diagnosed patients receiving radiation). The different forms of treatment-related heterogeneity that have resulted in radiographic challenges include (1) pseudoprogression, characterized by transient enlargement of the tumor with associated clinical symptoms, recently published for the authors’ DIPG cohort ( Fig. 2 A ) and recurrent low-grade glioma cohort ; (2) development of different types of noncystic and cystic focal signal abnormalities within the DIPG cohort and recurrent supratentorial high-grade glioma cohort (discussed later; Fig. 3 ); (3) development of both contiguous ( Fig. 2 B) and remote smaller lesions that eventually regress and/or undergo necrosis; and (4) one portion of the tumor responding to treatment while another portion of the tumor appears to be growing (see Fig. 7 ).
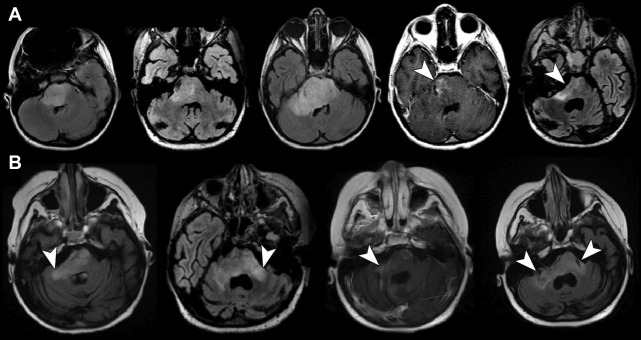
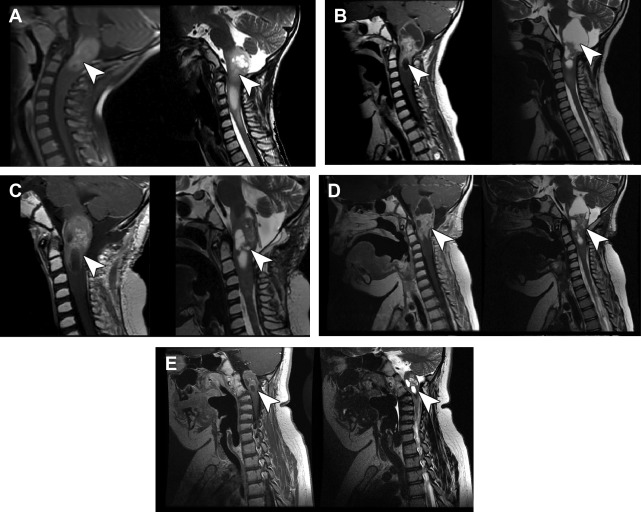
In a series of 21 children with DIPG treated with peptide-based vaccines at the authors’ institution, 4 children (19%) had documented pseudoprogression based on imaging and clinical criteria: 1 child had transient tumor enlargement in association with acute neurologic deterioration 4 months after beginning vaccination that later regressed and culminated in a sustained partial response (see Fig. 2 A) and 3 other children had symptomatic pseudoprogression, with transient neurologic deterioration and tumor enlargement followed by stabilization on decreasing steroid doses. After the episode of pseudoprogression, the patient with the subsequent partial response developed contiguous lesions in the bilateral middle cerebellar peduncle later in the course of peptide-based vaccine therapy. These lesions eventually underwent shrinkage and necrosis (see Fig. 2 B). Cases of pseudoprogression were also noted in other types of pediatric brain tumors treated with the peptide vaccine, including a cervicomedullary biopsy-proved anaplastic astrocytoma lesion (see Fig. 3 ), recurrent supratentorial high-grade gliomas, and recurrent low-grade gliomas.
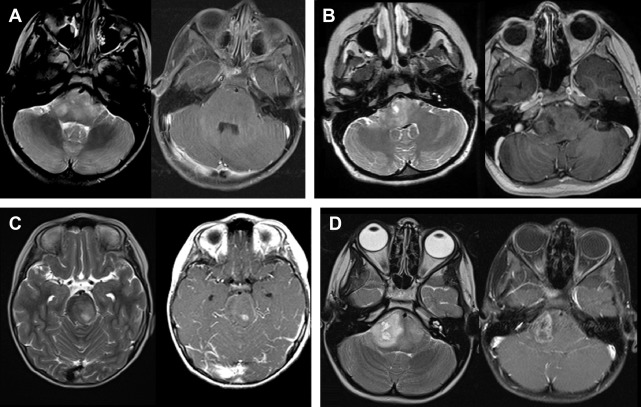
The authors also observed an unusually high incidence of focal cystic and noncystic signal intensity changes (likely representing evolving necrosis) in a pediatric DIPG population treated with the peptide-based vaccine. When these changes were classified into 4 categories based on T2 signal characteristics and postcontrast enhancement characteristics ( Fig. 4 ), 81% of these children developed focal areas of noncystic changes during immunotherapy, with an average time between starting vaccine to development of noncystic changes of 4.8 months (from 38 days to 10.8 months), and 57% developed focal cystic changes, with an average time of 6.5 months (from 1.2 months to 10.6 months) after the initiation of therapy. Of all the patients who developed cystic necrosis, 82% had noticeable enhancement in the region prior to the development of the necrosis. A small subset of patients had areas of enhancement that were stable or decreased in size on subsequent examinations. Studies are ongoing at the authors’ institution to correlate these patterns of focal signal abnormality with survival and pseudoprogression. These findings underscore the concept that conventional MR imaging has limitations in the ability to assess different forms of treatment-related imaging heterogeneity. The authors’ initial experience with the use of advanced neuroimaging modalities (ie, MRS, diffusion MR, and perfusion MR) to evaluate treatment response is described.
Magnetic resonance spectroscopy
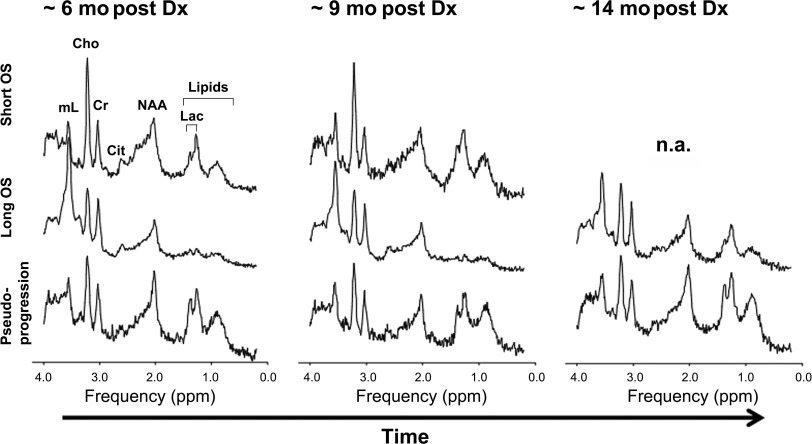
MRS provides a metabolic evaluation of the sampled tissue. In vivo intracellular metabolites with concentrations of 0.1 μmol/g to 0.5 μmol/g or higher can be assessed. Abnormal choline (Cho) metabolism is a common endpoint for many forms of cancer. Cho-containing metabolites are involved in the synthesis and breakdown of cell membranes. Because growing tumors require the net synthesis of cell membranes to support cell proliferation, the in vivo measurement of Cho provides surrogate information on tumor growth rates. Brain tumors generally have elevated levels of Cho, with higher Cho levels observed in more aggressive tumors. Another metabolic feature of aggressive tumors is a prominent signal from mobile lipids, although the time course is less predictable. Lipids (and lactate) can accumulate in cystic/necrotic areas but may also be recycled by tumor cells and/or surrounding cells for de novo cell membrane synthesis and for oxidation in the tricarboxylic acid cycle. Lipids may increase as tumors progress, for example, from grade III astrocytoma to glioblastoma. Myo-inositol is well regarded as a marker of gliosis as well as an important osmolyte whose regulation across the plasma membrane is a key cellular mechanism for mediating osmotic stress in astrocytes. In in vivo human studies, myo-inositol is consistently elevated in the setting of chronic inflammation, such as in multiple sclerosis and other neuroinflammatory central nervous system conditions, likely reflecting ongoing astrogliosis. Myo-inositol is generally elevated in ependymoma and gliomas, which are typically characterized by a high fraction of glial cells. Histopathologic studies have suggested there is increased reactive gliosis in individual tumors marked by an elevated myo-inositol concentration, and in a recent study, elevated myo-inositol distinguished tissue inflammation from tumor proliferation in adult glioblastoma patients treated with radiation therapy and adjuvant therapy. The authors are currently exploring the hypothesis that alterations in myo-inositol may be a predictor of outcome in certain forms of pediatric tumors treated with the peptide-based vaccines [in particular the DIPG cohort ( Fig. 5 )]. A key observation was the stability of serial MRS spectra in the setting of clinical and radiographic pseudoprogression (see Fig. 5 ). Specifically, the MRS spectra were stable comparing 3 time points of imaging: baseline, at the time of pseudoprogression, and after pseudoprogression. When the cases of pseudoprogression (eg, case shown in Fig. 2 ) were specifically looked at, there was serial stability in the Cho-to-creatine ratio, myo-inositol, and lipids/lactate (see Fig. 5 ). From these preliminary observations, the authors hypothesize that the stability of certain metabolite ratios (including Cho-to-creatine ratio) may distinguish treatment response and pseudoprogression from true progression in the setting of peptide-based treatment of high-grade gliomas (including DIPG). Likewise, stability in lipids/lactate and myo-inositol also may have the potential to distinguish pseudoprogression from true progression. These findings underscore the importance in obtaining serial MRS data at baseline and different points of therapy, including at the time of pseudoprogression. These preliminary observations need to be confirmed in large-scale multicenter studies.