Many viral infections can involve the central nervous systems (CNS) of fetuses, neonates, infants, and children. The pathogenesis, patterns of CNS involvement, and species of viral infection may differ in the developing fetus, infant and neonate, and early childhood. Familiarity with the clinical course and imaging appearances of the variable CNS diseases is helpful in making correct differential diagnoses and in prompting timely treatment. This article reviews the clinical courses, pathologic findings, and imaging features of the most common viral infections that may involve the CNS of neonates and infants, including congenital and neonatal CNS viral infections, common CNS viral infections, and parainfectious encephalomyelitis.
Many viral infections can involve the central nervous systems (CNS) of fetuses, neonates, infants, and children. The spread of the infections to the CNS may occur by way of the hematogenous route through the placenta, during the peripartum period, from the respiratory system, or the gastrointestinal tract, or through the peripheral nervous system. The pathogenesis, patterns of CNS involvement, and species of viral infection may differ in the developing fetus, infancy and neonate, and early childhood. For example, viral infections of the CNS in the fetus (particularly in the first and second trimesters) may cause severe destruction of the developing brain, thus leading to a developmental anomaly. In young children, parainfectious allergic encephalitis is often accompanied by typical clinical course and reversible (mostly) white matter demyelination in the brain and spinal cord, which share certain unique characteristics in the category of viral infection in children. Although different species of viral infection may have different patterns of CNS involvement, most viral encephalitides are nonspecific. Some viral infections may have recognized patterns of encephalitic lesion distribution, especially the herpes virus group (involving the gray matter) and the enterovirus (EV) group (involving the tegmentum of brainstem and spinal cord), which may help in imaging differentiation. In this article, the authors review the clinical courses, pathologic findings, and imaging features of the most common viral infections that may involve the CNS of neonates and infants, divided into the following three categories: congenital and neonatal CNS viral infections, common CNS viral infections in infants and young children, and parainfectious encephalomyelitis.
Congenital and neonatal central nervous system viral infections
Cytomegalovirus
Congenital cytomegalovirus (CMV) disease is the most common viral infection among newborns . In utero, transmission can come from primary maternal infection, or as a result of reactivation or reinfection of seropositive mothers. Infants can also be postnatally infected during parturition or after birth, from breast milk, saliva, urine, or other sources . Primary infection occurs in as many as 2.2% of pregnant women, and serologic or culture evidence of intrauterine CMV infection has been reported in 0.2% to 2.2% of all live-born neonates . The clinical symptoms and signs of the infected newborn include hyperactivity, hypotonia, jaundice, hepatosplenomegaly, petechiae, thrombocytopenia, small head size, seizure, chorioretinitis, impaired hearing, and developmental delay . Nearly one half of infected infants are reported to have CNS complications . Confirmative diagnosis requires viral isolation from the body fluid of infants within 3 weeks of birth or detection of CMV DNA from amniotic fluid (when intrauterine) or fetal blood by polymerase chain reaction (PCR) analysis, with the latter being reported 100% in sensitivity and 99% in specificity . The mechanisms of CNS injury in congenital CMV have been postulated to be the affinity of the CMV virus to the rapidly growing germinal matrix cells or lenticulostriate small vessels, resulting in abnormalities of the cerebral and cerebellar cortices, periventricular calcification, and lenticulostriate vasculopathy .
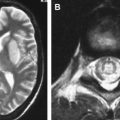
Transabdominal or transvaginal sonography is the first-line imaging study to identify brain abnormalities in fetuses with suspected CMV, followed by fetal MR imaging. The sonographic findings of congenital CMV infection include microcephaly, echogenic intraparenchymal foci (calcifications), ventriculomegaly, intraventricular adhesions, periventricular pseudocysts, sulcation and gyral abnormalities (lissencephaly and polymicrogyria), hypoplastic corpus callosum, cerebellar abnormalities (hypoplasia, calcification), large cisterna magna, and striatal vasculopathy . On CT and MR imaging, brain abnormalities depend on the degree of brain destruction and the timing of the injury. The clinical sequelae appear to be more severe if maternal infection occurs during the first or second trimester . The findings include lissencephaly (injury before 16 to 18 weeks of gestational age), polymicrogyria (injury between 18 and 24 weeks of gestational age) ( Fig. 1 ), extremely diminished volume of the white matter, delayed myelination, small cerebellum, enlarged lateral ventricles, and periventricular calcifications ( Fig. 2 ) .
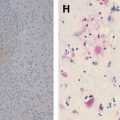
Herpes simplex virus
Neonatal herpes simplex encephalitis (HSE) is most commonly caused by the herpes simplex virus (HSV) type 2 (genital herpes); 85% of cases occur during the peripartum period, 10% in the postnatal, and 5% in utero. In patients 6 months of age or older, virtually all cases of HSE are caused by HSV type 1 (orofacial herpes) . Intrauterine HSE occurs in approximately 1 in 300,000 deliveries. Infants who acquired HSV in utero typically have a triad of clinical findings, consisting of neurologic (microcephaly, encephalomalacia, hydrocephaly, intracranial calcifications), ophthalmologic (microphthalmos, retinal dysplasia, optic atrophy, chorioretinitis), and cutaneous manifestations . Clinical manifestations include seizure, lethargy, irritability, tremors, poor feeding, temperature instability, and bulging fontanel . Mental retardation, severe neurologic deficits, or death may occur and the prognosis is usually poor . Clinical diagnosis can be made by detection of viral DNA by PCR of the cerebrospinal fluid (CSF) .
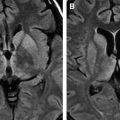
Unlike HSE in older children and adults, which usually involves the orbital surface of the frontal lobes, the temporal lobes, and insulae, with frequent hemorrhagic change, neonatal HSE often affects the cerebral white matter and, occasionally, the cerebellum . See the article by Dr. Bulakbasi elsewhere in this issue for further exploration of this topic. In the acute stage, CT may show patchy or widespread areas of hypoattenuated brain edema along with stippled high density, suggesting hemorrhage. On MR imaging, the brain edema appears as low signal on T1-weighted imaging and high signal on T2-weighted imaging, primarily involving the cerebral white matter that may progress to the cerebral cortex ( Fig. 3 A) . Rapid brain necrosis with hemorrhage can be seen as dark signal on T2-weighted images and susceptibility-sensitive gradient-echo images. The meninges may enhance after contrast administration . Diffusion-weighted MR imaging may show restricted water diffusion in the affected region earlier in the disease process, and, in some cases, is better than the conventional T2-weighted images or fluid-attenuated inversion recovery (FLAIR) sequences . Eventually, severe and diffuse cerebral atrophy with encephalomalacic change, punctuate or gyriform calcification, and ventriculomegaly may develop ( Fig. 3 B) .
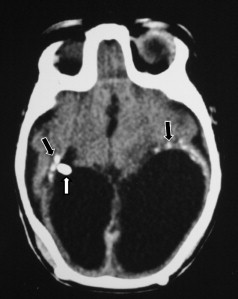
Rubella virus
Congenital rubella is a rare viral infection whose incidence has been reported to be less than 1 per 1 million live births in the United States . The most common clinical manifestations are deafness, ocular abnormalities (cataract, glaucoma, microphthalmos, chorioretinitis), cardiac anomalies (patent ductus arteriosus, pulmonary artery stenosis), and neurologic deficits (microcephaly, cerebral palsy, seizure, psychomotor retardation) when infection occurs in the first and second trimesters . The rubella virus is thought to inhibit the neuronal cell division directly and result in microcephaly . It also damages the blood vessels, leading to ischemia, necrosis, and calcification in gray and white matters, predominantly involving the periventricular white matter and basal ganglia .
Cranial ultrasound may reveal intraventricular strands and debris with periventricular echogenic foci, which are typical of ventriculitis . On CT study, low-density areas in the periventricular/subcortical white matter, microcephaly, ventriculomegaly, calcification over the periventricular white matter and basal ganglia may be seen, whereas MR imaging also shows delayed myelinization and oligo or macrogyria .
Common central nervous system viral infections in infants and young children
Varicella zoster virus
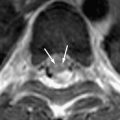
The congenital varicella zoster virus (VZV) infection is rare. Infection before the age of 20 gestational weeks may result in spontaneous abortion. VZV also causes chickenpox in childhood, after which the virus becomes latent in the cranial nerves, dorsal root, and autonomic nervous system ganglia. Reactivation results in herpes zoster (also known as shingles) in immunocompromised children and adults, most commonly distributed along 1∼3 thoracic dermatomes and the ophthalmic branch of trigeminal nerve. VZV reactivation from the geniculate ganglion (cranial nerve VII) can cause Ramsay Hunt syndrome, features of which include peripheral facial weakness and rash around the ear (zoster oticus) . CNS complications of varicella, including encephalitis, meningitis, and myelitis, affect less than 0.1% of children with chickenpox . The clinical symptoms and signs include fever, headache, altered consciousness, seizure, coma, ataxia, and focal neurologic deficits. Clinical diagnosis is based on typical skin vesicular rashes and detection of VZV antibody and DNA in CSF by PCR analysis . However, CNS involvement without the classic skin rash has been described . VZV can also spread to the blood vessels of the brain, producing a unifocal or multifocal vasculopathy (formerly called granulomatous arteritis) and complicating ischemic or hemorrhagic infarct ( Fig. 4 ) .
CT and MR imaging studies of varicella encephalitis may show abnormal density (signal intensity) and swelling of the cerebral cortex, basal nuclei, cortical-white matter junction or cerebellum (acute cerebellitis) . Conventional or MR angiography may show narrowing of the distal internal carotid artery and T-zone, indicating granulomatous arteritis .
Measles virus
The measles virus can involve the CNS in three different ways: acute postinfectious encephalitis, acute progressive encephalitis, and subacute sclerosing panencephalitis (SSPE) .
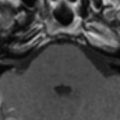
Acute measles encephalitis may be due to an autoimmune response, because the CNS has no detectable measles virus (viral antigen, RNA, or specific antibodies) . The incidence is about 0.1% among measles cases, with a mortality rate of 10% to 20% . Diagnosis is usually made based on clinical course, change in the titers of IgM and IgG antibodies to measles in the serum and CSF, and exclusion of other infectious or metabolic disorders . The onset of acute encephalitis is usually preceded by a prodromal febrile illness and cutaneous rash. After 1 to 11 days, impaired consciousness, generalized tonic-clonic seizure, weakness, and recurrent vomiting develop . Pathology shows perivascular inflammation and demyelination . The MR imaging study may show multifocal T2 hyperintensity in the bilateral cerebral hemispheres, both involving gray and white matter, with bilateral symmetric involvement of the putamen and caudate nucleus (striatal necrosis) . On diffusion-weighted imaging and apparent diffusion coefficient (ADC) maps, the lesions show cytotoxic edema in the acute stage . Gyriform contrast enhancement or hemorrhage may occur . It may lead to encephalomalacic change, and even brain atrophy, in as early as the third day of symptom onset .
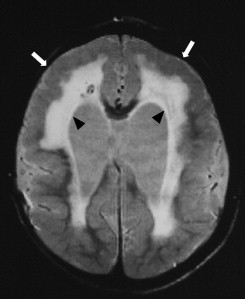
SSPE predominantly affects older children. The history of primary measles infection with a latent period of 6 to 8 years is found in most patients . The disease is incurable and usually causes death within 2 to 4 years of onset . The reasons for the long latency and the slow evolution of the disease remain unclear. The diagnosis is also based on clinical findings, EEG results, and titer of measles antibodies in the serum and CSF . Pathologic findings include neuronal loss, astrogliosis, demyelination, neurofibrillary tangles, and infiltration of inflammatory cells . The criteria of Jabbour used for clinical staging are as follows :
Stage I: personality change or behavioral disturbance, or both
Stage II: convulsive motor signs, myoclonus, incoordination, choreoathetosis, and tremor
Stage III: coma, opisthotonos, decerebrate rigidity, no response to any stimulus
Stage IV: loss of cerebral cortical function, less frequent myoclonus, diminished hypertonia
Early-stage SSPE (stage I, II) usually presents with normal MR imaging findings. In stage III, widespread periventricular T2 hyperintensity, starting from parieto-occipital lobes toward the frontal lobes, and cerebral and cerebellar atrophy may be found ( Fig. 5 ) . Lesions in the thalamus, basal ganglion, brainstem, and dentate nuclei of the cerebellum have also been reported . MR spectroscopy may detect early increased myo-inositol/creatine (possibly reflecting glial proliferation) and choline/creatine (possibly reflecting demyelination) ratios in stage II, whereas N-acetylaspartate/creatine ratio has been found to be normal in stage II and decreased in stage III (possibly reflecting neuronal loss) .
Enterovirus 71
EVs include poliovirus, echovirus, group A and B coxsackieviruses, and EVs 68 to 71 . Humans are the only natural hosts for EV, and the principal mode of human-to-human transmission is fecal-oral . EV 71 presents with the highest neurologic complication rates, which have been reported to be 50% . The most recent large outbreak, in 1998, affected thousands of children in Taiwan, with a fatality rate of about 14% . EV 71 causes hand-foot-mouth disease (fever, vomiting, ulcerative lesions in the oral mucosa, and vesicles on the dorsal surfaces of the hands and feet) and herpangina (small oropharyngeal vesicles and ulcers located at the anterior tonsillar pillar, soft palate, uvula, and pharyngeal wall), but sometimes has no cutaneous lesions . After an average of 3.2 days of prodromal symptoms, neurologic manifestations such as aseptic meningitis, acute flaccid paralysis (AFP), and rhombencephalitis occur . The diagnosis can be made by isolation of the virus or detection of viral DNA by PCR from CSF, throat swab, and stool specimens.
In rhombencephalitis, the symptoms can be graded as follows :
Grade I: myoclonic jerks and tremor, ataxia, or both
Grade II: myoclonic jerks and cranial nerve involvement (ocular disturbance, bulbar palsy)
Grade III: rapid onset of respiratory distress, cyanosis, poor peripheral perfusion, shock, coma, loss of doll’s eye reflex, and apnea
MR imaging shows T2-hyperintense lesions in the brainstem, most often in the pontine tegmentum, followed by medulla oblongata, midbrain, and dentate nuclei of the cerebellum ( Fig. 6 ) . In some cases, lesions in the thalamus and putamen were identified . Patients who have grade III symptoms may reveal brainstem atrophy and cavitation on follow-up images .
EV 71 is considered one of the leading causes of AFP, now that poliomyelitis has been nearly eradicated . Limb paralysis may also coexist with rhombencephalitis . AFP, defined as the acute onset of flaccid limbs and absent reflexes, may involve either unilateral or bilateral limbs . The symptoms may be indistinguishable from poliomyelitis, but AFP caused by EV 71 may present with myoclonus, tremor or ataxia, hand-foot-mouth disease, or herpangina, with a more benign course, either with complete recovery or mild residual motor weakness . MR imaging shows hyperintense lesions on T2-weighted imaging, with contrast enhancement on T1-weighted imaging involving the anterior horns of the spinal cord and ipsilateral ventral roots on the same side, or bilateral involvement (radiculomyelitis) ( Fig. 7 ) . The levels may be located from the cervical to the conus level of the spinal cord . Unilateral involvement is more common in the EV 71 infection, as compared with poliomyelitis, and has a better outcome than those with bilateral involvement .
Epstein-Barr virus
The Epstein-Barr virus (EBV) is known to be the causative pathogen of infectious mononucleosis (fever, pharyngitis, lymphadenopathy, hepatosplenomegaly, skin rash) in children. Neurologic complications, such as meningitis, encephalitis, acute disseminated encephalomyelitis (ADEM), cranial nerve palsy (most commonly involving cranial nerve VII), cerebellitis, myelitis, Guillain-Barré syndrome, and seizure, have been documented . The incidence of neurologic complications ranges from 0.4% to 7.3%, and CNS symptoms may be the only manifestations . Encephalitis and myelitis are each seen in fewer than 1% of patients who have acute EBV infection, resulting in neurologic deficits in 12% and mortality in 8% of cases . In pediatric patients, recent studies have found that EBV infection can be acute primary, reactivation, or chronic active infection . Diagnosis can be made by virus isolation or by the detection of anti-EBV antibodies or EBV DNA from serum and CSF specimens . Three major histopathologic patterns of EBV encephalitis have been reported: first, the edematous-hemorrhagic pattern; second, perivascular mononuclear infiltrates and occasional viral inclusions in cortical and subcortical cells; and third, perivascular lymphocyte inflammation with demyelination of white matter but no viral inclusions, suggesting ADEM equivalent .
On MR, acute EBV encephalitis typically may show T2-hyperintense lesions in the bilateral corpus striatum (caudate nucleus and putamen), with occasional involvement of the cerebral cortex, thalamus, splenium of the corpus callosum, and gray matter of the cervical cord (predominantly gray matter structures) ( Fig. 8 ) . The abnormal signal intensity lesions usually completely resolve on follow-up images .
HIV
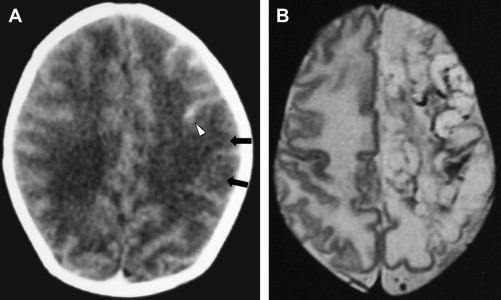
HIV causes AIDS. About 85% of AIDS cases in children were vertically transmitted from infected mothers; another mode of transmission is by blood transfusion, particularly in children with hemophilia . HIV appears to penetrate the blood–brain barrier early in the course of infection by way of infected macrophages, which bind avidly to the endothelial cells in the CNS . The prevalence of CNS disease in HIV-infected children ranges from about 20% to 60% . The onset of neurologic disease generally occurs between the ages of 2 months and 5 years . The neurologic complications of AIDS are protean, which may be caused by HIV itself (HIV encephalopathy); additional opportunistic infections (toxoplasmosis, CMV, mycobacteria, Cryptococcus neoformans, syphilis); or neoplasms (most commonly B-cell lymphoma associated with EBV infection) . Reactivation of latent John Cunningham virus because of a low CD4+ cell count also causes a demyelination disease known as progressive multifocal leukoencephalopathy (PML) . The miscellaneous infectious, neoplastic diseases and PML in AIDS children are similar to those in the adult group. They are discussed elsewhere in this issue.
HIV encephalopathy (or encephalitis) is a broad term that refers to the clinical deterioration of higher brain functions . It is divided into two types: (1) progressive encephalopathy, which is comparable to AIDS dementia complex; and (2) static encephalopathy, in which the children have better functions but do not keep up with the age-appropriate milestones . The most common abnormalities observed on images are brain atrophy and white matter disease . The three patterns of brain atrophy recognized on CT and MR imaging studies are (1) central atrophy due to preferential tropism of the virus for the basal ganglia and deep white matter causing necrosis and atrophy with disproportionate ventriculomegaly, as compared with cortical atrophy; (2) generalized atrophy, particularly affecting the frontal lobes; (3) necrotizing encephalopathy with encephalomalacia . The white matter lesions are better delineated on MR imaging than on CT. Pathology shows infiltration of the parenchyma and perivascular spaces by lymphocytes, and macrophages with reactive astrogliosis . The lesions tend to be located in the periventricular white matter and centrum semiovale, with no mass effect or contrast enhancement . Care must be taken in interpreting these lesions before age 2 because of the incomplete myelination. The white matter lesions tend to be more bilateral, symmetric, and diffuse, whereas the lesions in PML tend to be more focal, asymmetric, and common in the posterior parietal lobes .
Intracranial calcifications can be detected in about 33% of HIV-infected children . The calcifications are usually bilateral and symmetric, involving the globus pallidus and putamen ( Fig. 9 ) . The subcortical frontal white matter and cerebellum may occasionally calcify. Calcifications related to HIV infection are usually not seen before 10 months of age. If any exist, congenital infections such as toxoplasma, CMV, rubella, HSV, or syphilis, rather than HIV, must be considered .