Many viral infections of the nervous system cause stereotyped pathologic features and overlapping clinical and imaging features. Neuroimaging usually offers neuroanatomical localization of the pathology, degree of involvement of the nervous system, and response to therapy during follow up in a few instances. Neuroimaging is a useful adjunct for diagnosis.
Among the 61 known viral families, only a dozen contain members that cause central nervous system (CNS) disease. The viral genome contains either DNA or RNA, unlike bacteria and higher organisms, which contain both DNA and RNA. Lacking any form of motility, viruses spread using the environmental factors between the hosts. In the human host, they spread by using several routes like blood and cerebrospinal fluid (CSF) within infected leucocytes and via trans-axonal and trans-synaptic spread within the CNS. The signs and symptoms of most viral infections of the CNS are relatively nonspecific and are attributable to the inflammatory reaction of the host as a defense mechanism rather than to the virus itself. The neuropathologic manifestations of most viral infections fall into a limited number of stereotyped patterns, primarily depending on the specific virus, the age and immune status of the patient, the route of entry of the virus, and the coexistence of other neurologic diseases and infections altering the blood-brain barrier Table 1 and Box 1 show common viral infections involving the meninges, the gray matter and white matter and the clinical presentation, as a ready reckoner. The meningeal inflammation usually causes aseptic meningitis. On involvement of gray matter, viral polio encephalitis/poliomyelitis manifests with microglial reaction around infected neurons (neuronophagia) and perivascular lymphocytic cuffing. Some of the infections reveal characteristic intracytoplasmic or intranuclear inclusions based on whether the infection is caused by DNA or RNA virus ( Table 2 ). The viral infections exclusively involving the white matter like JC virus or HIV-1 elicit very little inflammation and present as demyelinating “encephalopathy”.
| Acute viruses | Subacute viruses (slow) |
|---|---|
| Poliomyelitis (RNA) | Progressive multifocal leucoencephalopathy (DNA) |
| Herpes simplex (DNA) | Herpes zoster (DNA) |
| Herpes zoster (DNA) | Rubella (RNA) |
| Measles (RNA) | Cytomegalovirus (DNA) |
| Rabies (RNA) | Subacute sclerosing panencephalitis (measles, RNA) |
| Echo (RNA) | HIV (RNA) |
| Coxsackie A and B (RNA) | Human T-cell lymphotropic (RNA) |
| Arboviral encephalitis (RNA) | Epstein-Barr (DNA) |
| Japanese encephalitis | |
| West Nile | |
| Dengue | |
| Nipah (RNA) |
Intra nuclear
Herpes simplex virus
Herpes zoster
Subacute sclerosing panencephalitis
Cytomegalovirus
Progressive multifocal leucoencephalopathy
Nipah virus
Intracytoplasmic
Herpes simplex virus
Rabies virus
Subacute sclerosing panencephalitis
Cytomegalovirus
Nipah virus
No inclusions
Japanese encephalitis virus
HIV (AIDS)
Human T-cell lymphotropic virus
Poliomyelitis
Epstein-Barr virus
Rubella
Cells involved
- •
Neurons
Herpes simplex virus, rabies, Japanese encephalitis virus, poliomyelitis, cytomegalovirus, Nepah virus
- •
Astrocytes
Cytomegalovirus, subacute sclerosing panencephalitis, herpes simplex virus, rabies (rarely), progressive multifocal leucoencephalopathy (rarely), rubella, Japanese encephalitis virus (in cultured cells?)
- •
Oligodendroglia
Subacute sclerosing panencephalitis, progressive multifocal leucoencephalopathy, herpes simplex virus
- •
Microglia
HIV (AIDS)
| Meningeal inflammation | Gray matter inflammation | White matter inflammation |
|---|---|---|
| Echo viruses | Enteroviruses | Progressive multifocal leucoencephalopathy |
| Coxsackie viruses | Polio | HIV, human T-cell lymphotropic virus |
| Enteroviruses | Coxsackie | HIV with highly active antiretroviral therapy |
| Herpes simplex virus 2 | Echoviruses | |
| HIV | Japanese encephalitis virus | |
| Mumps | West Nile virus | |
| Lymphocytic chorio-memingitis | Nipah virus | |
| Rabies |
The viral infections involving gray and white matter that produce panencephalitis may manifest as necrotizing or non-necrotizing forms. The necrotizing infections (eg, Herpes simplex encephalitis [HSE]) are associated with prominent mononuclear inflammatory cell reaction, including many foamy macrophages and lytic infection of neurons and glia and endothelial cells. Because of significant edema and tissue destruction, they mimic bacterial infections and ischemic and hypoxic lesions associated with vasculitidis. Non-necrotizing panencephalitis is the feature in cases of subacute sclerosing panencephalitis (SSPE), cytomegalovirus (CMV), HIV encephalitis, and human T-cell lymphotropic virus 1(HTLV-1) myelitis. The histology reveals sparse lymphocytic response, reactive astrocytosis, diffuse or nodular microglial reaction, and viral inclusions in neuronal/glial cells. Reactive inflammation in the CNS in cases of hypoxic, ischemic, and toxic brain damage may mimic non-necrotizing panencephalitis. The imaging features may not be useful in establishing definitive diagnosis, but they may assist in determining the promising site for brain biopsy if considered. Pathologic features of various viral infections are presented in brief to correlate with neuroimaging in neurologic practice.
Herpes simplex encephalitis
Herpes simplex virus (HSV) type 1 and 2 are DNA viruses, belonging to the herpes virus family. These groups of viruses, in addition to primary infection, are known to establish latency in sensory ganglia and cause recurrent disease manifesting as cutaneous eruptions by reactivation in an immunocompromised host. These viruses may manifest as commonly recognized cold sores, or less frequently as of herpetic keratitis, presumably related to the latency in trigeminal ganglia. HSV type 2 similarly establishes latency in sacral ganglia after primary genital infection and result in repeated episodes of genital herpes. Human herpes virus-6 is the etiologic agent for roseola in children and is associated with febrile seizures; human herpes virus-8 has been associated with Kaposi’s sarcoma in an immunocompromised state . The varicella-zoster virus (VZV) belongs to this family and is responsible for chicken pox; it also establishes latency in numerous cranial and spinal ganglia after the initial episode and may erupt as shingles in a dermatomal distribution corresponding to the ganglia affected. CMV, another virus of this family, essentially rests in reticuloendothelial cells and causes meningoencephalitis in neonates and adults. The ability of HSV types 1 and 2 to travel from the peripheral site of infection to the sensory ganglia to the brain and spinal cord was recognized following the pioneering experimental work of Good Pasture and Teague in rabbits .
HSV types 1 and 2 differ antigenically, differ in their DNA composition, and differ in in vitro cytopathic effect, though they both can cause acute necrotizing encephalitis in infants, children, and adults. Baringer and Pisani reported the latency of HSV and the presence of the viral genome in the CNS of patients who do not have obvious neurologic disease, especially in medulla, pons, and olfactory bulb (and occasionally in other areas). The pathogenetic association between latent HSV infections and HSV encephalitis is not well understood. The encephalitis caused by HSV-1 in children and adults is much more circumscribed and is limited to inferior frontal, medial temporal and adjacent insular cortex, and cingulate gyrus, while sparing the parieto-occipital lobes and cerebellum. Frequent involvement of the olfactory bulbs and tract has lead to the suggestion that the virus enters the CNS following infection of olfactory mucosa, which leads to the involvement of limbic structures . Comparison of viral DNA from the brain to oral herpetic lesions in patients who have HSV encephalitis revealed homology in only 50% of cases and nonidentity in the rest . Encephalitis caused by HSV-2 in newborn infants usually is wide spread in the brain and involves other organs like skin, eyes, and lungs.
HSV-1 easily can be cultured from the brain of patients, whereas successful isolation from the CSF is noted only with overwhelming infection. However, the viral genome can be amplified from the CSF by polymerase chain reaction (PCR) technology in a majority of HSV encephalitis cases .
HSV encephalitis in children and adults is the most frequent nonendemic cause of acute necrotizing encephalitis. The clinical presentation is similar to encephalitis produced by a variety of other endemic viruses like arboviruses, enteroviruses, varicella zoster, cytomegalovirus, Epstein Bar virus, rickettsial diseases, and toxoplasma encephalitis. Clinically, patients initially present with acute and subacute onset of headache, fever, behavioral abnormality, and focal or generalized seizures; they later develop dysphasia with involvement of dominant hemispheres and hemiparesis, reflecting deep structure involvement. Occasionally, patients may complain of olfactory and gustatory hallucinations with behavioral abnormalities, which strongly suggest the possibility of herpetic encephalitis. The characteristic electroencephalogram (EEG) features include periodic high voltage discharges predominating in one or both temporal lobes on a background of generalized slowing, which changes rapidly with progression of the disease. Diagnostic imaging studies have contributed greatly to our ability to recognize HSE and distinguish it from other encephalitis. The characteristic involvement of the medial temporal lobe, deep insular structures, subfrontal gyri (sparing the parieto-occipital areas and cerebellum) raise the index of suspicion of HSE. With progression of the disease, these lesions can be seen on CT scan and enhance with contrast. On MRI, the lesions are hypointense on T1W images, hyperintense on T2W, and enhanceed on contrast.
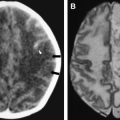
During the acute phase, HSE has stereotyped asymmetric pathologic lesions involving the temporal lobes, especially the anterior and inferior aspects, the insula, cingulate gyrus, and the posterior part of orbitofrontal cortex and the underlying white matter. In a majority of cases, one finds ischemic softening in the initial stages ( Fig. 1 A, B), which progresses to hemorrhagic lesions after 1 week, ( Fig. 1 C, D) which correspond to hyperintense lesions on a T2W image and contrast enhancement.
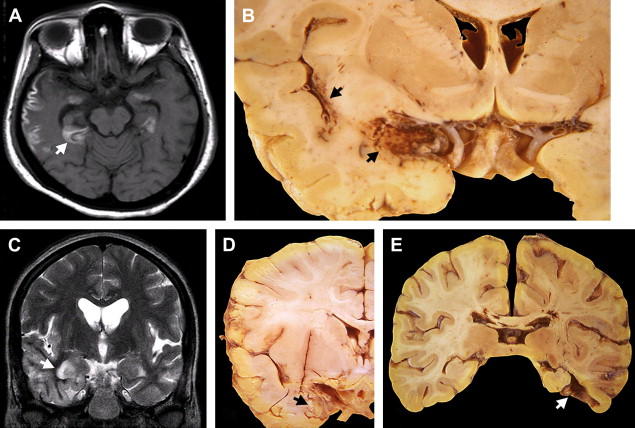
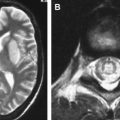
In patients who succumb to the disease many weeks after onset following partial treatment or untreated disease, the hemorrhagic necrosis progresses to pale, gliotic cavitation and atrophy with partial collapse of the affected temporal lobe and discolouration of the overlying meninges. In long-term survivors, especially those who were misdiagnosed with a psychiatric illness or dementia, the affected part of the brain appears shriveled and retracted, resembling a healed infarct ( Fig. 1 E).
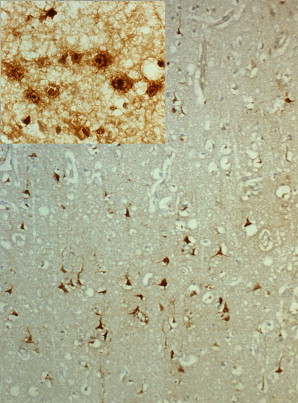
The histologic lesions of HSE extend from the pial surface, through the cerebral cortex, and into the white matter to a variable depth. In the initial stages, the parenchymal inflammation is minimal, and the polymorphs and lymphocytes accumulate around the blood vessels. The neurons, glia, and vascular endothelial cells in the brain biopsies look hypereosinophilic, and intranuclear eosinophilic inclusions in the cells are visible at the edge of the lesions. Occasional areas of active microglial nodules with neuronophagia may be seen as a feature of viral invasion. By using immunohistochemistry, the viral antigen can be localized in abundance in neurons and glia both in the cytoplasm and nuclei ( Fig. 2 ). The immunolabeled glial cells extend into subcortical white matter, the fiber tracts in the corpus collasum and internal capsule.
In hemorrhagic lesions, affected regions contain sheets of necrotic cells admixed with gitter cells and intense perivascular and interstitial lymphocytic infiltrates and apoptotic nuclei. Intranuclear inclusions may not be discernable except by immunohistochemistry, which reveals few cells with viral antigen. HSV antigen is demonstrable up to 3 weeks after the onset of symptoms , with the immunostained cell density gradually receding after the second week. Following treatment with acyclovir, the viral antigen demonstrable by immunohistochemistry becomes scarce. During the initial 2 weeks, the HSV nucleocapsid can be demonstrated by electron microscopy in the nuclei of the affected neurons and glia.
Pathologic lesions, especially those in children, resemble the ones found in VZV,CMV encephalitis and acute toxoplasma lesions. At times marked neuronophagia and pale acellular round lesions are found around vessels in the cortex, basal ganglia and thalamus resembling the ones seen in arboviral encephalitis and not distinguishable on histology. It is only seroepidemiology and PCR that can assist in establishing the diagnosis.
In patients dying in a few weeks after the onset with or without treatment, the histology reveals a cavity with variable number of haemosiderin containing histocytes, sidero-calcinotic neurons, a glial scar and occasional microglial nodule. The perivascular inflammation spilling into the parenchyma may be diffuse in the brain in addition to temporal lobe. The virus or its antigens are not demonstrable either by electron microscopy or immunohistochemistry. However the viral DNA can be demonstrated by multiplex PCR in the paraffin sections from the temporal lobe.
Occasional patients develop atypical form of HSE, especially following immunosuppression. These lesions are usually slowly progressive, significantly haemorrhagic involving the brainstem , containing viral antigen and viral DNA . Herpes myelitis is a rare complication of HSV infection, involving a few segments, with non-necrotising inflammatory process . HSV-2 occasionally causes ascending necrotizing myelopathy in immunosuppressed individuals .
Neonatal herpetic encephalitis is acquired in utero or during delivery by contact with infected maternal genitalia or during the early months by contact with infected secretions from the mother or others and it is common in premature infants . The encephalitic process can be localized to temporal lobe as in adults or diffuse involving the whole brain and other organs due to hematogenous spread the of virus. Infants with disseminated disease present at about one week of age with worse prognosis. The isolated/focal encephalitis presents usually after 2nd week after birth with non-specific clinical manifestations. The viral DNA can be demonstrated in CSF and serum. The brain on gross examination is swollen, congested, softened with parenchymal and ventricular hemorrhages. Long term survivors following acyclovir therapy show cystic encephalomalasia . Histology reveals diffuse necrotizing encephalitis with no anatomic predilection. During the initial days the viral inclusions, viral antigen and viral DNA are readily demonstrable and a few weeks later the viral DNA only by PCR. It is only the clinical suspicion that leads to the diagnosis of neonatal herpic lesions.
Human Herpes Virus 6 and 7 : Both viruses share genomic organization and biological properties, but antigenically distinct. The virus infection is acquired usually from mother by contact with infected saliva and breast milk . Both the viruses are CD4 tropic, which HSV-7, similar to HIV binds to CD4 receptor protein . The virus also infects monocytes, bone marrow progenitor cells and can be found in umbilical cord blood . Latent infection can be found on monocytes and cells in CNS . These viral infections are important cause of febrile seizures and may lead to cerebral atrophy . Rarely the primary infection causes meningoencephalitis with multifocal gray and white matter necrosis .
HSV-8 is associated with Kaposi’s sarcoma and lymphoma of lymphoreticular system . Symptomatic infection is associated with immunosuppression.
Varicella zoster infection
VZV is a DNA virus causing diffuse exanthematous cutaneous lesions of chickenpox in pediatric patients and focal dermatomal shingles in elderly patients. Morphologically, VZV is indistinguishable from HSV and has considerable molecular genetic homology . Primary VZV infection (varicella or chickenpox) is acquired by inhalation or mucosal contact with infected secretions. Following primary viremia, viral amplification occurs in the lymphoreticular system. Varicella infection of the CNS is rather uncommon and can manifest as aseptic meningitis, transverse or ascending myelitis, postinfectious encephalitis, or Guillain Barre syndrome in adults . In the immunocompromised, the incubation period is short and the cutaneous rash is more chronic, persistent or recurrent .
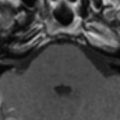
Varicella zoster infection establishes latency in dorsal root and cranial nerve ganglia, with the virus being transported along the axon . This viral infection is a relatively frequent complication of depressed cell mediated immunity . The principal VZV related CNS lesion is bulbar encephalitis or transverse myelitis associated with dermatomal ganglio radiculitis and rash. The pathologic lesions are nongranulomatous vasculitis and lead to single or multifocal infarcts ( Fig. 3 ), ventriculitis, and leptomeningeal vasculitis associated with haemorrhagic infarcts ( Fig. 4 ). None of these lesions have characteristic imaging features that aid in diagnosis.
Varicella zoster infection
VZV is a DNA virus causing diffuse exanthematous cutaneous lesions of chickenpox in pediatric patients and focal dermatomal shingles in elderly patients. Morphologically, VZV is indistinguishable from HSV and has considerable molecular genetic homology . Primary VZV infection (varicella or chickenpox) is acquired by inhalation or mucosal contact with infected secretions. Following primary viremia, viral amplification occurs in the lymphoreticular system. Varicella infection of the CNS is rather uncommon and can manifest as aseptic meningitis, transverse or ascending myelitis, postinfectious encephalitis, or Guillain Barre syndrome in adults . In the immunocompromised, the incubation period is short and the cutaneous rash is more chronic, persistent or recurrent .
Varicella zoster infection establishes latency in dorsal root and cranial nerve ganglia, with the virus being transported along the axon . This viral infection is a relatively frequent complication of depressed cell mediated immunity . The principal VZV related CNS lesion is bulbar encephalitis or transverse myelitis associated with dermatomal ganglio radiculitis and rash. The pathologic lesions are nongranulomatous vasculitis and lead to single or multifocal infarcts ( Fig. 3 ), ventriculitis, and leptomeningeal vasculitis associated with haemorrhagic infarcts ( Fig. 4 ). None of these lesions have characteristic imaging features that aid in diagnosis.
Cytomegalovirus
Though most adult humans are infected by CMV, only an immunodepressed host develops CNS pathology. The genomic organization and replication of CMV share similarities with the herpes virus. The infected cells, as the name indicates, are enlarged and have characteristic intranuclear and cytoplasmic inclusions. The virus spreads horizontally and vertically and can cross the placental barrier. From the site of primary infection, CMV reaches the brain hematogenously. Nearly all the effects of CMV on the CNS are directly caused by destruction of the infected cells. In immunocompetent patients, CNS involvement is rare and may manifest as meningitis, encephalitis, transverse myelitis, radiculomyelitis, and retinitis . CMV is the most common opportunistic infection in cases of AIDS . The CNS manifestations of CMV infection mimic those of HIV and other viral infections, and distinction becomes difficult ; CMV ventriculitis usually is asymptomatic even when severe. Relatively uncommon CMV myelitis and peripheral neuritis could be caused by CMV infection of vascular endothelial cells and ischemia or autoimmune hypersensitivity reaction .
Neonatal symptomatic disease is caused by infection in utero; the risk of transmission of primary maternal infection is 40%–50%, which is unlike a reactivated disease with a risk of 2% . Postnatal infection by contact with infected secretions and breast milk usually is subclinical. Severe forms of congenital infection manifest as microcephaly or chorioretinitis, which leads to death. Surviving children may have microcephaly, mental retardation, spasticity, hearing loss, optic atrophy, and occasionally hydrocephalus . In milder cases, deafness and minimal brain dysfunction with intellectual and behavioral dysfunction may manifest in later life. Postnatal infection of premature children manifests with severe systemic manifestations .
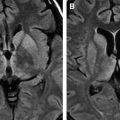
Neonatal cytomegalic inclusion disease is wide-spread. The brain is usually small, showing porencephaly, polymicrogyria, cerebellar hypoplasia, and hydrocephalus with focal periventricular calcification. Histology reveals meningoencephalitis with inclusions in neurons, glia, choroid plexus, and vascular endothelial cells. Postnatal infection is characterized by widespread microglial reaction, and nodules and inclusions are uncommon . In adults, the virus causes low-grade encephalitis with diffuse microglial response. In patients who have AIDS, the disease may accompany HIV encephalitis and other herpetic infections . In AIDS patients, CMV can cause necrotizing encephalitis and ventriculitis ( Fig. 5 ). Numerous inclusions are found in denuding ependyma and subependymal glial cells, sparsely involving the neurons. Associated inflammatory response in parenchyma and around vessels is sparse.
In view of the ubiquitous nature of the virus and high seroprevalence, only quantitative PCR of the CSF may help in identifying CMV encephalitis .
Epstein-Barr virus
The Epstein-Barr virus (EBV) was identified first in cultures of Burkitt’s lymphoma . Though found in various tumors, the role of EBV viral DNA in genesis of neoplasms is uncertain still . The spread of this viral infection occurs through nosopharyngeal contact with infected saliva, leading to infectious mononucleosis and involvement of the reticuloendothelial system. CNS involvement is rare, with features of mild nonspecific meningoencephalitis. Association of this viral infection with multiple sclerosis is debated still .
Hendra and Nipah virus infections
The Hendra and Nipah viruses belong to the paramyxovirus group. The Hendra virus was recognized first in Australia , and the Nipah virus was recognized first in Peninsular Malaysia and Singapore . Both viruses cause necrotizing meningoencephalitis. Pathologic examination reveals widespread vasculitis, thrombosis and ischemic infarcts, and a presence of endothelial syncitia, eosinophilic intraneuronal inclusions, and microglial nodules. Like other forms of viral encephalitis, MRI may show focal hyperintensities in deep nuclear areas .
Progressive rubella panencephalitis
Rubella infection in children and adults causes acute exanthematous German measles, with nearly 50% remaining subclinical . The congenital infection results from transplacental spread of the virus from maternal infection. In the brain, astrocytes are more susceptible to infection than neurons and oligodendroglia. In congenital rubella, the virus can persist within the CNS and can be isolated from the CSF even after 1 year . The most common neurologic abnormalities are sensorineural deafness, psychomotor retardation, and encephalitis. Grossly, the brain is usually small, associated with hydrocephalus, acqueductal stenosis, polymicrogyria, and neural tube defects. Histology shows collagenous thickening of vessels and mineralization of the wall in deep white matter. Foci of parenchymal necrosis and lymphohistiocytic reaction can be seen close to affected vessels, indicating ischemic origin rather than primary viral encephalitic process .
Progressive rubella encephalitis, similar to SSPE, manifests several years after the initial, congenital infection. The main pathology is gliosis and gelatenous change of white matter, wide-spread neuronal loss and gliosis in gray matter, perivascular lymphocytic cuffing, and microglial nodules. No viral inclusions are demonstrable. The consistent feature is deposition of Periodic Acid Schiff (PAS)-positive amorphous and mineralized material on the medium sized vessels.
Measles encephalitis: subacute sclerosing panencephalitis
The measles virus, a single stranded RNA virus belonging to the paramyxovirus family, manifests clinically in two forms of subacute encephalitis: measles inclusion body encephalitis and subacute sclerosing panencephalitis.
Measles inclusion body encephalitis develops within a few months of exposure to measles in patients who have a depressed immune system . Similar to SSPE, the clones of the measles virus show mutations in the genomic sequence encoding for M, H, and F viral proteins with restricted or abnormal expression of these proteins . In view of depressed cell mediated immunity, the disease has rapid course and has abundant viral inclusions in neural cells in contrast to SSPE. The patients manifest myoclonus and neurologic deficits. The brain, upon imaging and gross examination, appears relatively normal. Histology reveals numerous intranuclear and cytoplasmic eosinophilic inclusions in neurons, astrocytes, and oligodendroglia, admixed with microglial reaction. Electron microscopy and immunohistochemistry reveal the viral nuclearcapsid and the viral antigen. Perivascular inflammation is sparse, and occasional multinucleated giant cells containing intranuclear viral antigen may be found.
Subacute sclerosing panencephalitis
SSPE is a disease of patients who have no immunologic impairment, manifesting usually a few years after the initial measles infection, which contrasts with measles inclusion body encephalitis. The risk of SSPE is diminished significantly by prior vaccination and is enhanced several fold if measles is contacted in the first year of life . In SSPE, there is hypermutation of the M, H, and F proteins and clonal expansion of the mutated virus . In view of defective budding of the virus to the cell surface, the virus accumulates in the cell. As the cell fusion function of F and H proteins are preserved, the virus spreads diffusely by fusion and also forms multinucleated giant cells . Because the virus is not exposed, it evades the immune response, facilitating clonal expansion of mutated virus despite antibody response to the structural proteins being strong.
SSPE presents between 5–15 years of age, with the average interval between initial measles infection and manifest SSPE being 7 years, unlike inclusion body encephalitis . SSPE progresses from initial symptoms of intellectual impairment and behavioral abnormalities to motor impairment, myoclonus, ataxia, spasticity to final stage of stupor, autonomic impairment, and coma. The progression of the disease is variable, lasting from a few months to years.
Neuroimaging reveals white matter changes. The brain on gross examination can be normal or atrophic related to the duration of the illness. Corresponding to changes on imaging, the white matter may be firm in texture and granular ( Fig. 6 A, B).