, John Go2, Robert W. Henderson2, Paul Kim2 and Meng Law3
(1)
Rancho Research Institute, Downey, CA, USA
(2)
Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
(3)
Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Case 12.1: Langerhans Cell Histiocytosis
History
29-year-old female with palpable neck mass.
Findings
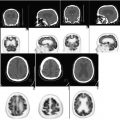
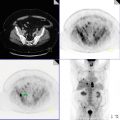
Axial post-contrast CT (Fig. 12.1a), FDG-PET (Fig. 12.1b), and PET-CT fusion (Fig. 12.1c) demonstrate hypermetabolic, peripherally enhancing mass inseparable from the posterior parotid gland.


Fig. 12.1
Impression
Biopsy-proven Langerhans cell histiocytosis (LCH) .
Pearls and Pitfalls
Differential diagnosis for parotid masses is broad including benign and malignant etiologies. The finding in this case represents involvement of an intra-parotid lymph node. The most common site of involvement by LCH is the bone (90 %) followed by the skin (30 %). FDG-PET is sensitive in detection of multifocal disease and shown to be superior to technetium 99 m methylene diphosphonate bone scans or radiograph in detection of active osseous lesions and response to therapy. There are limited number of studies evaluating utility of FDG-PET in soft tissue involvement by LCH.
Discussion
LCH is a rare idiopathic “neoplastic” process secondary to monoclonal proliferation of Langerhans-type cells. It is more common in the pediatric population, with a peak incidence between 1 and 3 years of age. There is also a male predilection (M:F 1.2–2.1:1). The course of the disease ranges from spontaneous regression to rapid progression (especially common in young children with multisystem disease). LCH can involve multiple organ systems, single-organ system with multiple sites, or single site. Diagnosis is confirmed histologically by tissue sampling.
The prognosis is variable and depends on disease burden, with single-organ system disease carrying better prognosis than multisystem disease. In 2008 the WHO recommended distinguishing LCH from a more pleomorphic variant know as Langerhans cell sarcoma, which carries a worse prognosis. Treatment ranges from excision or limited radiation for single-focus disease to systemic chemotherapy and steroid administration for multifocal disease and supportive care in cases of endocrine and CNS involvement (Fig. 12.1).
Case 12.2: Crossed Cerebellar Diaschisis
History
43-year-old female with remote history of biopsy-proven oligoastrocytoma within the right cerebral hemisphere status post radiation.
Findings
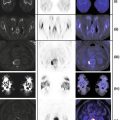
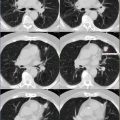
CT: Areas of calcification with associated volume loss and no appreciable mass effect centered in the right thalamus and internal capsule compatible with known previously treated oligoastrocytoma (Fig. 12.2).


Fig. 12.2
FDG-PET: Relative decreased FDG uptake in the right cerebral hemisphere as compared to left compatible with post radiation change. Relative decreased FDG uptake in the left cerebellar hemisphere as compared to right compatible with crossed cerebellar diaschisis (Fig. 12.2).
MRI: Subtle asymmetric left cerebellar volume loss with no decreased diffusion or signal abnormality (Fig. 12.2).
Impression
Crossed cerebellar diaschisis.
Pearls and Pitfalls
Crossed cerebellar diaschisis refers to hypometabolism in a cerebellar hemisphere contralateral to a cerebral hemispheric lesion. Lesions located in the motor cortex, anterior corona radiata, and thalamus (as in this case) produce the most marked suppression of the contralateral cerebellar cortical metabolism. Findings of hypometabolism are seen on FDG-PET. Decreased blood flow to the contralateral cerebellum has also been shown on MR perfusion imaging in setting of MCA territory stroke. In setting of chronic hypometabolism, cerebellar atrophy ensues.
Discussion
Crossed cerebellar diaschisis (CCD) has been reported in patients with ischemic and hemorrhagic hemispheric stroke, during carotid amytal procedure, migraine attack, seizure, space occupying lesion, and focal volume loss of a cerebral hemisphere. CCD is thought to be due to interruption of corticopontocerebellar fibers and subsequent transneuronal metabolic and blood flow alterations that are distant to and on the opposite side of the primary lesion. Border-zone cerebellar infarcts have also been reported in setting of CCD especially in migraines with prolonged aura. In setting of cerebral infarct, CCD is associated with poor clinical outcome (Fig. 12.2).
Case 12.3: Tumor Recurrence/Progression
History
43-year-old female with remote history of treated oligoastrocytoma WHO grade III, within the right cerebral hemisphere status post radiation and chemotherapy about 14 years ago with increased area of enhancement on surveillance MRI (prior MRI is not shown here). Clinical concern for tumor recurrence/progression versus radiation necrosis.
Findings
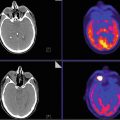
PET-CT: Focal mass-like area on CT shows abnormal avid FDG uptake which is increased in extent and avidity as compared to prior studies (not shown) (Fig. 12.3).


Fig. 12.3
MRI: Approximately 2 cm area of new mild enhancement (prior study not shown) and increased cerebral blood volume (CBV) on dynamic susceptibility contrast-enhanced cerebral blood volume (DSCE-CBV) magnetic resonance imaging (i.e., MR perfusion) with associated decreased diffusion.
PET-MRI: Overlap of abnormality on PET and MRI confirming hypermetabolism and increased CBV (Fig. 12.3).
Impression
Tumor recurrence/progression.
Pearls and Pitfalls
In setting of treated glioma, differentiating radiation necrosis from tumor recurrence/progression on imaging is crucial but often difficult. Findings on conventional imaging are relatively nonspecific and often unable to distinguish the two entities. Utility of fluorodeoxyglucose positron emission tomographic (FDG-PET) , which studies tumor metabolism, for differentiation of glioma recurrence/progression from radiation necrosis has been controversial with reported specificities as low as 18 %. However, considering recent advances in MR perfusion imaging, which studies tumor vascularity, combination of the two modalities maybe helpful. These imaging tools have also been for differentiation of high-grade from low-grade tumors and benign lesions.
Discussion
Primary central nervous system neoplasia is one of the most frequent causes of death between 15 and 35 years of age. Gliomas constitute >90 % of primary brain tumors diagnosed after the second decade of life. Despite treatment with chemotherapy and radiation, including proton-beam therapy (PBT) and other radiosurgery, the majority of these tumors progress and/or recur. Moreover, treatment with radiation remains associated with tissue necrosis that may also lead to clinical deterioration. Differentiating recurrent tumor (or progression of tumor) from predominant radiation necrosis has treatment and prognostic implications.
Oligoastrocytomas histologically represent mixed glial cell origin, astrocytoma, and oligodendroglioma with a peak incidence occurring in the third to fifth decade. The incidence among males is higher than females. Prognosis is similar to anaplastic astrocytoma and worsened with increased age of onset, with 5-year survival of greater than 50 % and a 10-year survival of 25–34 %. On histologic evaluation, predominance of oligodendrocytes over astrocytes confers a better prognosis. Treatment is controversial and includes surgical reduction, radiotherapy, and chemotherapy often with corticosteroids and seizure prophylaxis. In our case, surgery was not possible as the tumor was in an eloquent location (Fig. 12.3).
Case 12.4: Stage IVa (T2 N2a M0) Oropharyngeal/Tonsillar HPV-Positive Squamous Cell Carcinoma
History
42-year-old male with no history of smoking or alcohol consumption presents with neck mass.
Findings
Axial post-contrast CT (A), PET (B), and PET-CT fusion (C) and coronal post-contrast CT demonstrate 2.5 cm FDG-avid mass involving the left palatine tonsil/oropharynx with associated FDG-avid 5.4 cm level 2–4 lymph node (Figs. 12.4 and 12.5). There is also nonspecific FDG uptake in the right palatine tonsil and both sublingual glands, none of which were involved by tumor.



Fig. 12.4

Fig. 12.5
Impression
Stage IVa (T2 N2a M0) oropharyngeal/tonsillar squamous cell carcinoma.
Pearls and Pitfalls
Squamous cell carcinoma (SCC) accounts for the vast majority of malignancies of the oral cavity and oropharynx and is commonly evaluated with radiologic imaging. The symptoms of disease, the routes by which it may spread, and the prognosis vary greatly, depending in large part on the anatomic site at which the primary tumor originates and the stage of the tumor at time of presentation. FDG-PET-CT is used at most centers for detection of otherwise occult primary oropharyngeal SCC (OSCC), as well as determining the local extent and stage of the tumor (including lymph node and distant metastasis). In our case, the tumor was about 2.5 cm with a 5.4 cm ipsilateral lymph node and no distant metastasis, making it T2, N2a, M0, or stage IVA [1]. Tables 12.1 and 12.2 describe TNM staging of oropharyngeal SCC [1].
Table 12.1




TNM staging of oropharyngeal squamous cell carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree