American College of Radiology NI-RADS is a surveillance imaging template used to predict residual or recurrent tumor in the setting of head and neck cancer. The lexicon and imaging template provides a framework to standardize the interpretations and communications with referring physicians and provides linked management recommendations, which add value in patient care. Studies have shown reasonable interreader agreement and excellent discriminatory power among the different NI-RADS categories. This article reviews the literature associated with NI-RADS and serves as a practical guide for radiologists interested in using the NI-RADS surveillance template at their institution, highlighting frequently encountered pearls and pitfalls.
Key points
- •
NI-RADS is a helpful surveillance imaging template that standardizes interpretations and communication with referring physicians.
- •
NI-RADS scores provide a level of suspicion for recurrence of head and neck cancer with linked management recommendations.
- •
ACR NI-RADS templates are available for CECT, MR imaging, and PET/CT.
- •
NI-RADS lexicon and scores have been linked with outcomes and shown to provide discriminatory power in detection of head and neck cancer recurrence.
- •
Use of NI-RADS templates allows the radiologist to add value in patient care.
Introduction/background
The American College of Radiology (ACR) published its first Reporting and Data System (RADS) for breast imaging in 1993 and, since that time, there have been several additional ACR-endorsed RADS systems that have emerged, including computed tomography (CT) colonography, coronary artery disease, liver imaging, lung, neck imaging (NI-RADS), adnexal masses, prostate imaging, and thyroid imaging. These RADS systems are devised by a consensus group of experts, and help to :
- •
Provide a standardized terminology, report organization, and assessment classification, typically used when there is a binary outcome for the interpretation of the study (ie, is there cancer or not).
- •
Reduce ambiguity in reports, linking management recommendations with an assessment classification, which provides an actionable report for the referring provider.
- •
Simplify communication, clearly directing management via a multidisciplinary approach.
- •
Facilitate radiologic-pathologic correlation for quality improvement.
- •
Foster evidence-based practice by providing data-minable reports for future studies.
- •
Add value to the radiologist, because they help to move patient-centered care forward.
NI-RADS was first introduced in 2015 as a novel surveillance imaging template for head and neck cancer. Head and neck primary malignancies recur in up to 15% to 50% of cases, most commonly in the first 2 to 3 years after treatment, and therefore surveillance imaging is important during that time frame to attempt to identify recurrence as early as possible, allowing for increased options for salvage treatment. Post-treatment changes to the already complex anatomy of the head and neck can complicate the interpretation of post-treatment imaging, and in the setting of free text reporting, can lead to a varied and suboptimal interpretation, which often depends on the preferences and experience level of the interpreting radiologist. NI-RADS standardized reporting templates provide a standard nomenclature and approach for interpreting these complex examinations, allowing for improved communication between radiologists and their referring clinicians, and standardization across institutions.
This article serves as a practical guide to using the NI-RADS approach and system for interpreting these complex post-treatment head and neck cancer studies. We review the different templates and legends for the different imaging modalities, providing case-based examples for guidance on assigning categories, and highlighting important commonly encountered pearls and pitfalls.
Imaging technique/algorithm
There are few evidence-based guidelines and standards regarding the optimal timing and modality of imaging surveillance. The National Comprehensive Cancer Network recommends baseline post-treatment imaging “within 6 months of treatment” for advanced head and neck primary cancers but does not officially recommend imaging for asymptomatic patients after 6 months. However, 79% of surveyed head and neck surgeons admit to using imaging surveillance on asymptomatic patients. PET combined with contrast-enhanced CT (CECT) has been shown to have good sensitivity and moderate specificity for detecting recurrent or persistent disease in the post-treatment setting; however, the optimal timing and frequency has not been standardized. , Beyond the initial baseline examination, there are few studies demonstrating a clear benefit or survival advantage for universal surveillance imaging. However, a recent study used NI-RADS data to look back at asymptomatic recurrence rates and determined that more than one-third of recurrences detected on imaging were clinically occult, and 80% of those occurred beyond the 6-month post-treatment timepoint. Given the high recurrence rates and frequent difficulty clinically assessing the primary site and neck for recurrence caused by post-treatment changes, most institutions have adopted a routine surveillance protocol for advanced head and neck cancers. Developing a consensus surveillance imaging algorithm at your institution with the stakeholders involved is important before using the NI-RADS imaging template. Standardizing the approach and the template also helps to study optimal imaging protocols for certain high-risk patient populations in the future.
In the NI-RADS white paper published by the Journal of the American College of Radiology in 2018, the ACR NI-RADS committee suggested the following baseline surveillance imaging regimen for head and neck squamous cell carcinomas:
- •
Baseline post-treatment PET/CECT at 8 to 12 weeks after completion of definitive therapy (has been shown to have a high negative predictive value at this timepoint).
- •
If the initial study is negative, CECT of the neck alone (or PET/CECT) 6 months later.
- •
If this is negative, repeat CECT of the neck alone 6 months later.
- •
If second follow-up study is negative, then CECT of the neck and chest performed 12 months later.
If there are suspicious findings on imaging (NI-RADS scores 2 or higher, as discussed later), more frequent imaging or intervention may be necessary. Because 95% of asymptomatic recurrences occur within the first 24 months after treatment, routine PET/CT surveillance after this time may be of limited value. , Although NI-RADS was initially developed for use with PET and CECT imaging, the ACR committee recommends the use of templates for MR imaging as well, because MR imaging is often best for assessing intracranial, perineural, and/or orbital disease in patients with certain primary tumors, including sinonasal, nasopharyngeal, orbital, or parotid malignancies, or other skull base tumors with propensity for perineural tumor spread. Finally, although most head and neck primary malignancies requiring surveillance imaging are certainly squamous cell carcinoma, this template can be adopted and applied to any head and neck primary malignancy.
Neck imaging reporting and data system template and legend
The post-treatment neck imaging templates are streamlined and efficient to use ( Box 1 ). The radiologist enters the initial stage, subsite, human papilloma virus status, and prior treatment completion dates in the Clinical Indication section. The technique can be prepopulated, and dates of comparisons added. The body of the report includes sections devoted to postsurgical change, if applicable, and postradiation changes (ie, laryngeal mucosal edema, subcutaneous stranding, and thickening of the skin), and a section dedicated to the primary site and to the neck, looking for local residual or recurrent disease or for metastatic cervical lymphadenopathy, prepopulated with normal findings. Thus, in a routine study, with no suspicious findings for recurrence, which is a default for the template, it is quick for the radiologist to tab through the sections and quickly finalize the report. In the setting of suspicious findings, it is easy for the radiologist to modify the respective section to add specific findings.
INDICATION:[ ]
Subsite & human papilloma virus status:[ ]
Surgery & chemoradiation:[ ]
TECHNIQUE:
COMPARISON:[<None.>]
FINDINGS:
[<No evidence of recurrent disease is demonstrated at the primary site.>]
[<No pathologically enlarged, necrotic, or otherwise abnormal lymph nodes.>]
Expected post-treatment changes are noted including [<supraglottic mucosal edema and thickening of the skin and subcutaneous soft tissues.>]
There are no findings to suggest a second primary in the imaged aerodigestive tract.
Evaluation of the visualized portions of brain, orbits, spine, and lungs show no aggressive lesions suspicious for metastatic involvement.
IMPRESSION:
Primary: [1]. [<Expected post-treatment changes in the neck without evidence of recurrent disease in the primary site.>]
Neck: [1], [<No evidence of abnormal lymph nodes.>]
In the Impression section, the primary site and the neck are each individually assigned a category from 0 to 4, based on the radiologist’s level of suspicion regarding the possibility of recurrence. There is a legend at the bottom of the report linking the category of the primary site and the neck to a management recommendation, based on literature-based best practices and consensus opinion of the ACR NI-RADS committee. The categories are as follows:
- •
Category 0: New baseline study. Need prior imaging for comparison to score (if prior imaging does exist and will become available for comparison).
- •
Category 1: No evidence of recurrence. Linked management recommendation is for routine follow-up.
- •
Category 2: Low suspicion, ill-defined areas of abnormality, with only mild enhancement or mild fluorodeoxyglucose (FDG) uptake. Linked management recommendation is for direct inspection of superficial or mucosal abnormalities, or short interval follow-up of deeper abnormalities with imaging.
- •
Category 3: High suspicion, discrete, new, or enlarging lesion with marked enhancement and/or intense focal FDG uptake. Linked management recommendation is for biopsy.
- •
Category 4: Definitive recurrence, either pathologically proven or definite radiologic or clinical progression.
Reporting templates, including the legends with management recommendations included at the end of the report, are slightly different for CECT/MR imaging and PET ( Box 2 and 3 ). Current lexicons are specific for CECT and PET findings; however, MR imaging lexicon verbiage has been suggested in the literature , and will likely be published by the ACR NI-RADS committee in the future. If there are findings that are worthy of the impression beyond the assessment of the primary site or nodal basin (ie, lung nodules suspicious for metastasis, severe atherosclerotic disease, or bone metastasis), additional findings are added at the bottom of the impression.
Primary
- 1.
No evidence of recurrence: routine surveillance
- 2.
Low suspicion
- a.
Superficial abnormality (skin, mucosal surface): direct visual inspection
- b.
Ill-defined deep abnormality: short interval follow-up a or PET
- a.
- 3.
High suspicion (new or enlarging discrete nodule/mass): biopsy
- 4.
Definitive recurrence (path proven, clinical or definitive imaging progression): no biopsy needed
- 1.
Nodes
- 1.
No evidence of recurrence: routine surveillance
- 2.
Low suspicion: (enlarging lymph node without morphologically abnormal features): short-interval follow-up or PET
- 3.
High suspicion (new or enlarging lymph node with morphologically abnormal features): biopsy if clinically needed
- 4.
Definitive recurrence (path proven, clinical or definitive imaging progression): no biopsy needed
- 1.
a Short-interval follow-up: 3 mo at our institution
Primary
- 1.
No evidence of recurrence: routine surveillance, CECT
- 2.
Low suspicion
- a.
Superficial abnormality (eg, skin, mucosal surface): direct visual inspection
- b.
Ill-defined deep abnormality with only mild FDG: short-interval follow-up a or repeat PET/CECT at routine follow-up
- a.
- 3.
High suspicion (new or enlarging discrete nodule/mass with intense FDG uptake): biopsy
- 4.
Definitive recurrence (path proven or clinical progression): no biopsy needed
- 1.
Nodes
- 1.
No evidence of recurrence: routine surveillance
- 2.
Low suspicion (residual nodal soft tissue with only mild/intermediate FDG): short-interval follow-up or repeat PET/CECT at routine follow-up
- 3.
High suspicion (new, enlarging with intense FDG uptake): biopsy
- 4.
Definitive recurrence (biopsy proven or clinical progression): no biopsy needed
- 1.
a Short-interval follow-up: 3 mo at our institution
{Distant disease} : optional if interpreting whole-body PET, same 1–4 categories
Neck imaging reporting and data system categories
The specific categories with their imaging description, lexicon for image interpretation, and pearls and pitfalls are discussed in detail below. In the report template, a category is assigned separately for the primary and the neck.
Neck Imaging Reporting and Data System category 0: incomplete assessment
Primary Site
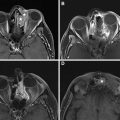
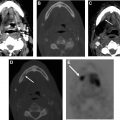
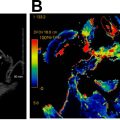
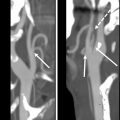
A NI-RADS 0 designation is made for the primary site when there are post-treatment changes on a new baseline study, with no prior imaging available for comparison; however, there is knowledge that prior examinations do exist and could potentially become available for review at a later time, at which point an addendum with an updated score/management recommendation should be provided. This score should be used rarely, and in most settings with post-treatment changes, but no suspicious features, the radiologist should feel confident giving an NI-RADS 1. However, there are instances when this assessment is helpful. In the author’s institutional experience, this category is most commonly assigned in the setting of post-treatment MR imaging NI-RADS assessments of sinonasal, nasopharyngeal, or any other tumor with skull base or perineural involvement, where there is persistent abnormal soft tissue, and it is difficult to determine if it represents sterile treated tumor versus persistent or progressive active disease ( Fig. 1 ). Additionally, this denotation is helpful in the setting of unusual tumors or in follow-up MR imaging to obtain the pretreatment imaging to determine if abnormalities are similar to the pretreatment appearance of tumor.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree