Fig. 8.1
Edwards Sapien 3 transcatheter aortic valve (Figure used with permission from Edwards Lifesciences Corporation, Irvine, CA, USA)
The initial ante-grade access is still available as trans-apical procedure, the trans-septal access however has been completely abandoned. At present the retrograde trans-femoral access is clearly predominant (Fig. 8.2). Occasional patients with a difficult femoral access are also done via a subclavian, direct aortic or carotid retrograde approach.
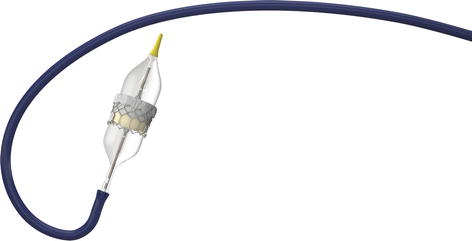

Fig. 8.2
Transfemoral delivery system “commander” for the Edwards Sapien 3 transcatheter valve (Figure used with permission from Edwards Lifesciences Corporation, Irvine, CA, USA)
Initial use of TAVR was restricted to elderly patients with high and very high surgical risk assessed by surgical risk scores as the Euroscore or the STS score [4, 5, 28]. It became clear that especially the Euroscore overestimated the surgical risk that is better predicted by the STS score [5, 29] and the Euroscore II [30]. However, STS and Euroscore II also neglect important risk determinants like frailty [31] Individual patient assessment by heart teams is therefore essential for patient selection.
Meanwhile two randomized landmark trials have confirmed the efficacy of TAVR with first generation transcatheter valves in patients with prohibitive surgical risk compared to medical therapy and in high risk patients compared to surgical valve replacement (SAVR). In the cohort B of the PARTNER trial 358 patients with aortic stenosis who were not considered to be suitable candidates for surgery underwent randomization to TAVR with the Edwards Sapien valve or standard therapy. Mean age of the cohort was 83 years and logistic Euroscore >20 %. One year mortality in the TAVR group was 30.7 % compared to 50.7 % in the group with standard therapy (p < 0.001) [11]. In the cohort A of the PARTNER trial 699 high-risk patients (logistic Euroscore >20 %) with a mean age of 84 years were randomized to TAVR or SAVR. One year mortality in both groups was comparable (24.2 % for TAVR and 26.8 % for SAVR). The rate of stroke and vascular complications was higher with TAVR whereas major bleedings and atrial fibrillation occurred more often after SAVR [32]. The second first generation transcatheter valve, the CoreValve, was implanted in the U.S. CoreValve trial. The extreme risk group was a single arm registry of 506 patients with aortic-stenosis and prohibitive risks for surgery which showed a promising 24.3 % mortality and 4.3 % stroke rate after 1 year [33]. The high risk group consisted of 795 patients with a mean age of 83 years and a logistic Euroscore of approximately 18 %. Patients were randomized to TAVR with the Corevalve or SAVR. Mortality after 1 year was significantly lower in the TAVR compared to the SAVR cohort (14.2 % vs 19.1 %; p = 0.04 for superiority) with no increased stroke rate (8.8 % versus 12.6 %) [34].
The better results of TAVR in the U.S. CoreValve trial compared to the PARTNER trial might be multi-factorial. Randomized data comparing different valve types are limited [35]. In spite of the fact that specific endpoints for TAVR trials have been defined (VARC and VARC II criteria) the short re-design cycles of these devices quickly invalidates such comparisons at present.
Paravalvular leaks, non-retrievability and access size have been felt to be the main shortcomings of the first generation devices. Paravalvular leaks have been associated with worse outcomes [36]. New device designs seem to be able to effectively reduce paravalvular leaks [37, 38]. Also other shortcomings are addressed [35].
Randomized comparisons of different access routes are lacking. Therefore any comparison of trans-apical and trans-femoral access is based on biased registry data with equivocal results. Patients’ preference however seems to go for least invasive procedures with percutaneous closure of access site in conscious sedation. A transfer of TAVR therapy into lower risk populations is discussed very controversially.
The need for antiplatelets after the procedure for patients not on anticoagulation is not well defined. Many centres tend for long-term aspirin treatment and sometimes addition of clopidogrel for various time periods.
Transcatheter Valve Repair for Mitral Insufficiency
The most extensive experience on transcatheter therapy of mitral insufficiency exists for the MitraClip device (Abbott; Menlo Park, California) which is used for direct valve repair. In addition many other interventional approaches have been introduced including different annuloplasty procedures and transcatheter mitral valve replacement. The future relevance of these latter approaches is not yet clear. Therefore they are beyond the scope of this description.
Mitral insufficiency can originate from a primary abnormality of the valve, degenerative mitral regurgitation (DMR), or a functional abnormality secondary to left ventricular dysfunction, functional mitral regurgitation (FMR). In functional mitral regurgitation the underlying disease might be ischemic or non-ischemic. Obviously a long-standing DMR can cause left ventricular dysfunction leading to secondary FMR which results in a mixed situation.
Current American guidelines recommend surgery (especially mitral valve repair) for symptomatic patients with chronic severe mitral insufficiency due to a primary valvular abnormality as a class I indication whereas surgery for symptomatic patients with severe functional mitral insufficiency should only be considered as a class IIb indication [13]. The latter recommendation is based on the fact that the reported outcomes in this cohort of patients were equivocal.
The MitraClip system was developed on the basis of Alfieri’s surgical edge-to-edge repair [39]. The clip (Fig. 8.3) is delivered percutaneously from the femoral vein via a transseptal sheath under fluoroscopic and transoesophageal echocardiographic guidance. It reduces mitral regurgitation by approximating the anterior and posterior leaflets thus creating a double mitral valve orifice (Fig. 8.4). If necessary two or even more clips can be delivered in one session.
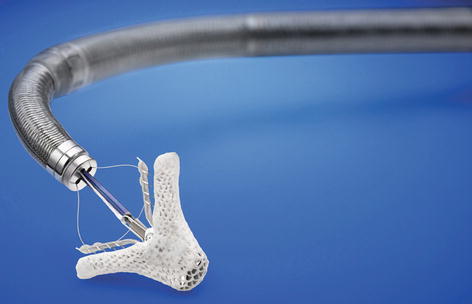
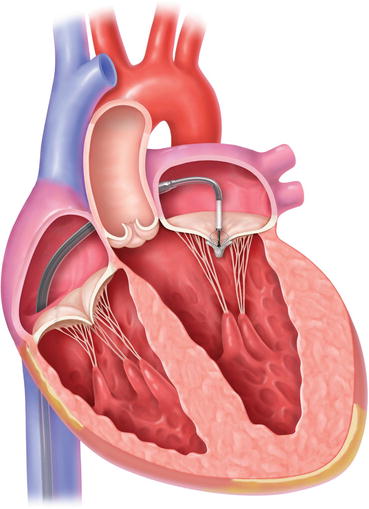

Fig. 8.3
MitraClip system for reduction of mitralregurgitation by approximating the anterior and posterior leaflets (Figure used with permission from Abbott Vascular, Santa Clara, CA, USA)

Fig. 8.4
Mode of implantation of the MitracClip system via transfemoral access (Figure used with permission from Abbott Vascular, Santa Clara, CA, USA)
After safety and feasibility of the MitraClip system had been shown in the first in men single arm EVEREST I trial [40] it was compared in the multicentre, randomized EVEREST II trial against surgery (either replacement or repair) in patients with symptomatic severe mitral regurgitation who were also candidates for mitral valve surgery [41]. FMR was only present in 27 % of patients. Patients with severe LV dysfunction were excluded. Mitral regurgitation and NYHA class were significantly reduced in both groups. However, in the as treated analysis surgery more effectively reduced mitral regurgitation than the MitraClip (4 % vs 19 % residual high degree mitral insufficiency). Major adverse cardiac events after 30 days were comparable between groups. After 4 years 25 % of patients in the percutaneous repair group vs 5.5 % in the surgical group had undergone redo surgery for significant mitral regurgitation [42].
Thus the MitraClip is no alternative to surgery in patients who are low-risk candidates for surgery but in those who are deemed to be at high surgical risk. This was confirmed in several registries and applied to degenerative as well as functional etiologies [43, 44].
Recently a pooled analysis of 16 studies with a total of 2980 patients of whom 2689 were considered high-risk for surgery showed a low incidence of procedural death (0.1 %). Thirty day mortality was 4.2 % which compares very favourably to historical surgical data.
Atrial Septal Defect Closure
Patients with significant atrial septal defects (ASD) frequently remain asymptomatic until adulthood. A majority only develops symptoms beyond the fourth decade. Therefore freedom from symptoms (e.g. reduced functional capacity, exertional shortness of breath, palpitations due to supraventricular arrhythmias, right heart failure) is no reliable predictor of a further benign course even in an elderly patient. A significant left to right shunt is usually defined as a pulmonary to systemic flow ratio (QP: QS) > 1.5. This ratio is however not only dependent on defect size but also on the LA to RA pressure gradient which is influenced by right and left ventricular compliance as well as pulmonary resistance. Left to right shunt can increase with decreasing left ventricular compliance (e.g. due to co-morbidities like arterial hypertension) or decrease with increasing pulmonary resistance. So a QP: QS < 1.5 does not exclude a significant ASD with preceding higher shunt volume. Therefore the 2010 ESC guideline on the management of grown-up congenital heart disease recommend to define a significant shunt rather based on signs of right ventricular volume overload [45]. A defect diameter of less than 10 mm with signs of right heart failure should however always raise suspicion on a causal relationship. In these cases an abnormal pulmonary venous connection has to be excluded.
It has been shown that surgical closure of significant ASD’s beyond the age of 40 years might have a small mortality but definitely a good symptomatic effect compared to medical treatment [46]. Mortality after transcatheter closure is comparable to surgical closure. Morbidity is however higher with the surgical approach [47, 48]. This might be even more pronounced in the elderly.
Defect closure does not lower the frequency of arrhythmia during follow up in the elderly patient [46, 49]. If ablation of atrial fibrillation or flutter is considered this might therefore be performed before defect closure. Peri-interventional atrial fibrillation is however frequently self-limiting.
European guidelines recommend closure of all atrial septal defects with significant shunt as defined by right ventricular volume overload regardless of symptoms and as long as pulmonary vascular resistence is still less than 5 Wood units. ASD occlusion should also be considered if paradoxical embolism is suspected. No special recommendation is given for the elderly. Transcatheter closure is the preferred method if technically feasible. In patients with modestly increased pulmonary resistance an individualized decision has to be taken considering the response on vasodilators (preferably nitric oxide) [45].
A number of different devices suitable for transcatheter closure of atrial septal defects has gained CE mark over the recent years. A detailed technical description of all the different devices would be beyond the scope of this book. The only device which gained full approval for clinical use from the United States Food and Drug Administration (FDA) is the Amplatzer Septal Occluder (ASO). It is also the device with the most complete study results. The ASO is a self-expandable double-disk device made of nitinol which is connected by a 3–4 mm waist between the two disks with the left disk being larger than the right. A total of three Dacron polyester patches is sewn into each disk and the connecting waist. The device size is determined by the diameter of its waste and currently available from 4 to 38 mm. The device is released from a venous sheath which is advanced from the right atrium via the defect into the left atrium. After release of the left atrial disk the waste is released in the defect which self-centres the device before release of the right atrial disk (Fig. 8.5).
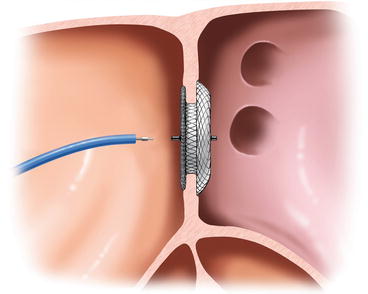

Fig. 8.5
Release of the Amplatzer Septal Occluder (Figure used with permission from St. Jude Medical GmbH, St Paul, MN, USA)
Transcatheter closure can only be considered in secundum ASD, which makes up for about 80 % of all ASD’s. With primum ASD (15 %), superior sinus venosus defect (5 %), inferior sinus venosus defect (<1 %) and unroofed coronary sinus as well as in patients with abnormal pulmonary venous connections a surgical closure has to be performed. Before transcatheter closure of secundum ASD a transoesophageal echo is mandatory to look for the pulmonary venous connection and the tissue rim around the atrial septal defect. The rim should be at least 5 mm of adequate quality except towards the aorta. In addition the defect should not be bigger than 36–38 mm [45]. Interventional closure of large ASD’s requires special technical skills and equipment (e.g. Hausdorf sheath).
When closing ASD’s in elderly patients with more co-morbidities special consideration should be given to impaired systolic or diastolic function. The acute volume challenge of the left ventricle after the closure might increase the end-diastolic pressure to an extent that pulmonary oedema results. Precautions should be taken when the atrial filling pressure before closure is above 15 mmHg. A test occlusion of the ASD with the sizing balloon can be performed monitoring the atrial pressures via the wire lumen over 10–15 min. If the left atrial pressure increases by more than 5 mmHg closure should be postponed and patients pretreated with diuretics and after-load reduction before a further closure attempt with initial repetition of the left atrial pressure measurements. In some cases a fenestrated occluder might be considered [50].
In elderly in whom no interventional closure can be performed an individual risk benefit analysis should be performed before surgical closure taking into account the individual co-morbidities [45].
After the procedure a minimum of 6 month aspirin treatment at a dose of 100 mg is recommended. Many centres add 75 mg clopidogrel for a variable time. Endocarditis prophylaxis is recommended during the first 6 month after device implantation [45].
Left Atrial Appendage Closure
The concept of left atrial appendage (LAA) closure for stroke prevention in atrial fibrillation is based on the observation that more than 90 % of emboli seem to originate from the left atrial appendage [51]. Surgical closure with different techniques have been done for several years; nevertheless the mainly non-randomized data were equivocal [52, 53].
Technical feasibility of interventional occlusion via a transseptal sheath was first shown in 2001 with the dedicated Plaato device which is no longer commercially available [54]. Shortly afterwards occlusion procedures with non-dedicated Amplatzer devices were reported [55]. These first procedures were predominantly done in patients with contra-indications to oral anticoagulation. After the procedure patients received dual antiplatelet therapy with aspirin and clopidogrel for 1–6 months usually followed by lifelong aspirin. With respect to the non-negligible bleeding risk also aspirin was stopped in some patients after 6 months.
Basically two different scenarios for LAA occlusion have to be considered: as alternative to anticoagulation when oral anticoagulation is possible and as replacement for anticoagulation when anticoagulation is not possible.
Actually only the first concept was tested in a randomized fashion with the dedicated Watchman device in the PROTECT AF and the PREVAIL studies. In spite of early hazards due to peri-procedural complications equivalence to oral anticoagulation with warfarin could be shown after 1065 patient years of follow-up in 707 patients with respect to the combined primary endpoint of stroke, cardiovascular death and systemic enbolisation (3.0 vs 4.9 events per 100 patient years) and superiority after 2621 patient years of follow-up (2.3 vs 3.8 events per 100 patient years) in the PROTECT AF trial [56, 57]. The interventional LAA occlusion in the PROTECT AF trial was followed by warfarin treatment for another 45 days which should be replaced by clopidogrel up to 6 month if transoesophageal echo did not show thrombus formation on the device. Warfarin and clopidogrel were given in combination with aspirin that was recommended for lifelong therapy. After 45 days and 2 years a vast majority of patients in the device group (87 % rsp. 94 %) had actually abandoned warfarin therapy. The PROTECT AF results were confirmed by the PREVAIL trial which also showed a significant reduction of peri-procedural complications (e.g. perforation, stroke, device embolization) compared to the PROTECT AF trial probably due to increasing operator experience [58]. Non-surprisingly the average age of the studied population in both trials was above 70 years what reflects the increasing prevalence of atrial fibrillation and embolic stroke risk with age.
A current consensus statement from the European Society of Cardiology is still reluctant to generally recommend LAA occlusion as alternative to anticoagulation when anticoagulation is possible [59]. The main arguments are that the early interventional hazards with device closure are significant especially at the beginning of the learning curve which is deemed to be rather slow compared to other interventions. Furthermore the main advantage of LAA closure compared to vitamin K antagonists seems to be the reduction of hemorrhagic strokes which might also be achieved with the novel oral anticoagulants that have not been compared yet to LAA device occlusion. Nevertheless the Watchman device recently gained approval for this indication from the United States Food and Drug Administration (FDA).
The focus of the current European consensus statement is rather on replacement of anticoagulation when anticoagulation is not possible. It has to be clearly stated that this indication is only based on registry data and non-randomized comparisons to historical stroke-rates or calculated stroke-rates according to established risk scores. Of the four CE marked devices the Watchman, the ACP including the newest Amulet generation and the Wavecrest aim at a mechanical device obstruction of the LAA via a purely endocardial approach whereas the Lariat device is used for a endocardial/epicardial suture ligation. Most data are available for the Watchman and the ACP (Fig. 8.6). For both devices registries have been performed without post-interventional anticoagulation but dual antiplatelet therapy for 1–6 months followed by indefinite aspirin therapy [60, 61]. Cessation of aspirin after 6 months was also reported [59].