Non-Hodgkin Lymphoma
Todd M. Blodgett, MD
Alex Ryan, MD
Omar Almusa, MD
Key Facts
Terminology
Non-Hodgkin lymphoma (NHL)
NHL: Malignancy of B or T lymphocytes
Imaging Findings
Spleen involved in 20% of patients with NHL
Other extranodal lymphoma may arise in CNS, peripheral nervous system, lung and pleura, bone, skin, breast, testis, and GU tract
PET/CT: Use with contrast-enhanced CT for staging
Enlarged LN, extranodal mass with low to moderate FDG uptake
Enlarged/normal-sized FDG-avid nodes in liver or spleen; “misty mesentery”
PET excellent for predicting prognosis in aggressive NHL after therapy
Post-therapy evaluation
Absence of metabolic activity on FDG PET following treatment (high predictive value for disease-free survival)
Persistent metabolic activity on FDG PET following treatment (moderate predictive value for recurrence)
Top Differential Diagnoses
Normal Structures
Reactive Lymph Node Hyperplasia
Sarcoid
Histoplasmosis
Other Malignancy
Diagnostic Checklist
Perform a baseline PET/CT in all patients with newly diagnosed NHL
TERMINOLOGY
Abbreviations and Synonyms
Non-Hodgkin lymphoma (NHL)
Large B-cell lymphoma
Low grade follicular B-cell lymphoma (FL)
Small lymphocytic lymphoma (SLL)
Chronic lymphocytic leukemia (CLL)
Marginal zone lymphoma (MZL)
Mucosa-associated lymphoid tissue (MALT)
Cutaneous T-cell lymphoma (CTCL)
Post-transplant lymphoproliferative disease (PTLD)
Hodgkin disease (HD)
Definitions
NHL: Malignancy of B or T lymphocytes
Low grade lymphoma
Slow growing, indolent non-Hodgkin lymphoma
Low grade diffuse B-cell lymphoma
SLL: Termed CLL when 1° in blood or marrow
Lymphoplasmacytic lymphoma (immunocytoma)
MZL: MALT; nodal marginal zone; splenic marginal zone
FL
Small-cleaved cell type: < 20-25% large cells
Mixed small-cleaved and large cell type: Survival inversely proportional to large cell percentage
Large cell type: Intermediate grade but more aggressive in nature
CTCL: Mycosis fungoides (MF), Sézary syndrome (leukemic variant)
CTCL: Rarely transforms into more aggressive large cell lymphoma
Separate entity from aggressive peripheral T-cell lymphoma (PTL)
Also separate from adult T-cell lymphoma/leukemia (ATLL)
Extranodal lymphomas
Refer to lymphomas located in Waldeyer throat ring, thymus, and spleen
IMAGING FINDINGS
General Features
Best diagnostic clue
Pre-therapy evaluation
High grade NHL: Multiple enlarged lymph nodes or nodal groups with intense FDG activity ± splenic/other organ involvement
Low grade NHL: Enlarged LN, extranodal mass with low to intense FDG uptake
Marked FDG uptake may represent high grade transformation
“Misty mesentery” also a common finding in NHL
Occasionally normal-sized FDG-avid nodes
PET/CT Post-therapy evaluation
Absence of metabolic activity on FDG PET following treatment (high predictive value for disease free survival)
Persistent metabolic activity on FDG PET following treatment (moderate predictive value for recurrence)
Location
Superior mediastinal and paraaortic nodes common
NHL known for less predictable spread than HD
Head and neck region
Second most common site of NHL
Primary head and neck lymphoma accounts for 10-20% of all cases of NHL
Prone to be asymptomatic and unsuspected clinically
Usually presents on PET/CT as asymmetrical intense FDG activity in a lymphoid structure
Common locations: Palatine, lingual, sublingual tonsils, and adenoids
Pulmonary
Pulmonary involvement uncommon
Involvement without nodal disease seen more commonly in recurrent disease than at presentation
Also seen more commonly in PTLD
Bone marrow
PET/CT can help direct bone marrow biopsy to most metabolically active areas
Frequently have abnormal focal FDG activity without a correlative CT abnormality
Involvement found in 50-80% of low grade NHL and 25-40% of high grade NHL
Tends to signify advanced stage disease
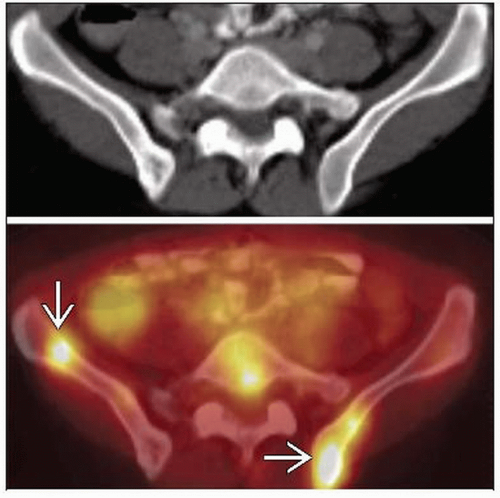
Spleen
Involved in 20% of patients with NHL
Defined as nodal in HD, extranodal in NHL
Organ size is poor predictor; spleen can be large but not involved or normal with infiltration
PET/CT significantly more accurate than CT alone for detecting splenic involvement
May appear as multiple lesions or diffuse involvement on FDG PET
Other extranodal lymphoma
CNS, peripheral nervous system, lung and pleura, bone, skin, breast, testis, and GU tract
GI lymphoma represents approximately 10-15% of all NHL
Size
Most patients being evaluated for new onset lymphoma will have enlarged nodes
PET/CT will often detect additional normal-sized but malignant nodes
Lymph node size is a poor predictor of tumor involvement
Imaging Recommendations
Best imaging tool
PET/CT
Preferred modality for staging NHL
Sensitivity/specificity for evaluating malignant nodes: PET/CT 91%, 90% vs. CT 88%, 86%
Sensitivity/specificity for extranodal involvement: PET/CT 88%, 100% vs. CT 50%, 90%
Current and potential clinical applications of FDG PET/CT
Initial diagnosis: Evaluate adenopathy ± systemic symptoms without current pathologic diagnosis of NHL
Determine staging
Guide biopsy
Assess conversion to higher grade
Evaluate early response to chemotherapy
Determine restaging
Monitor post-treatment progress
Conventional imaging staging technique
CECT of neck, chest, abdomen, pelvis; occasionally MR
Uni- or bilateral bone marrow biopsy
Protocol advice
Consider PET/CT with contrast-enhanced CT for staging
Stage with PET/CT before any therapy is administered
One dose of chemotherapy may ↓ FDG uptake
Consider low dose CT for restaging/surveillance after a negative PET/CT following therapy
CT Findings
NECT
Enlarged lymph nodes
Can be large conglomerate masses with lobulated margins
Calcification rarely seen prior to treatment; frequently seen after therapy in larger confluent nodal masses
Size is not an independent predictive factor
CECT
Slight to moderate uniform enhancement following IV contrast
Marked enhancement unusual (low attenuation in 20% of cases)
Masses from lymphoma more likely to encase and displace the mediastinal structures
Unusual to constrict or invade them
Lung/Mediastinum
Intrathoracic involvement in 50% of newly diagnosed cases (vs. 85% in HD)
20% present with mediastinal adenopathy
Single or multiple discrete pulmonary nodules less well-defined and less dense than carcinoma
May cavitate (10-20%)
Consolidation with air bronchograms (solitary or multiple, includes pseudolymphoma)
Diffuse reticulonodular opacities (lymphocytic interstitial pneumonia)
Post-obstructive atelectasis due to nodal compression
Pleura
Pleural effusions seen in 10% of patients at presentation, due to lymphatic or venous obstruction
Effusion, may resolve with irradiation of mediastinal lymph nodes
Pleural masses rare
Pericardial
Pericardial effusion mostly coexists with adenopathy adjacent to pericardial margins
Associated with high grade peripheral T lymphoma, large B-cell lymphoma, and PTLD
Chest wall
Invasion with rib destruction uncommon
Nuclear Medicine Findings
Initial diagnosis
PET/CT used to evaluate enlarged nodes in patients without a history of lymphoma
Covered indication by CMS, but rarely used for initial diagnosis
Some NHL may not be FDG avid
MALT generally less FDG avid, but as a group MALTs have complex histology and may demonstrate uptake
PET for differentiation of indolent vs. aggressive lymphoma
Controversial topic
SUV ≥ 10 confers higher likelihood for aggressive disease (considerable overlap exists)
Staging
PET/CT more sensitive than CT for staging NHL
Consider baseline PET/CT for all patients with newly diagnosed NHL
Consider directed bone marrow biopsy to most metabolically active osseous structures detected on PET
Splenic involvement much more accurately assessed with PET/CT than with CT alone
Liver involvement may present as diffuse disease with patchy infiltrates originating in portal areas
Other patterns include miliary with multiple small lesions or, rarely, large focal lesions
False negative bone marrow biopsy may result due to patchy nondisseminated marrow involvement
Bone marrow biopsy (BMB) alone can detect minimal bone marrow disease, which may escape detection by PET
PET/CT shown to modify radiotherapy planning in 44% of patents with head/neck lymphoma
CLL/SLL: PET of limited use in staging 2° ↓ FDG uptake (sensitivity 58%)
SUV > 3.5 suggests Richter transformation of CLL/SLL → diffuse large B-cell lymphoma (sensitivity 91%, specificity 80%, PPV 53%, NPV 97%)
MZL: FDG PET staging sensitivity 71% (lower for extranodal)
MALT lymphoma: Typically no or low FDG uptake; SUV > 3.5 suggests plasmacytic differentiation
CTCL: FDG PET useful in staging, especially in suspected single cutaneous site
CTCL: Intense nodal sites suspicious for large cell transformation
FL: FDG PET useful in staging all grades (sensitivity 94%, specificity 100%)
Wide overlap between FDG uptake by lower (SUV 2.3-13) and higher grade (SUV 3.2-43) FL
Emergence of sites of ↑ FDG uptake (SUV > 10): Transformation to higher grade (specificity 81%)
Upstaging of extranodal disease observed mostly in stage I and II disease
Response to therapy
FDG PET demonstrates poor sensitivity for predicting likelihood of response/progression in patients with indolent lymphoma
PET excellent for predicting prognosis in aggressive NHL after therapy
Usefulness of follow-up scan hinges on existence of pre-therapy scan indicating FDG-avid disease
Early identification of therapy response allows modification of ineffective treatment
Nonresponders may avoid unnecessary side effects
Tumor FDG uptake decreases dramatically as early as first week post-treatment in aggressive NHL
Strong predictive value for 18 month outcome when imaged early in chemotherapy cycle, after only one cycle
FDG PET in early response assessment (after 1-4 cycles): Sensitivity 79%, specificity 92%, PPV 90%, NPV 81%, accuracy 85%
FDG PET in post-Rx assessment (mixed population HD, NHL): Sensitivity 79%, specificity 94%, PPV 82%, NPV 93%, accuracy 91%
FDG PET in post-Rx assessment NHL: Sensitivity 67%, specificity 100%, PPV 100%, NPV 83%, accuracy 88%
FDG PET in post reinduction chemo (before stem cell transplant): Sensitivity 84%, specificity 83%, PPV 84%, NPV 83%, accuracy 84%
1999 European Organization for Research
Post-therapy SUV that increases 25% over baseline indicates progressive disease
SUV decrease of 15-25% after cycle 1 of chemotherapy and 25% after more than 1 cycle indicates partial metabolic response
Ga-67-citrate less sensitive and specific than FDG PET for aggressive lymphomas
Chemotherapy can cause marrow hyperplasia and also generalized FDG uptake
G-CSF and recombinant erythropoietin can result in diffusely increased FDG uptake bone marrow and spleen, limiting sensitivity
Uptake due to growth factors usually returns to baseline by one month post-therapy
Restaging
Rationale for FDG PET imaging post-therapy
Allow for treatment of residual/progressive disease before it spreads further
PET can be positive months before histological confirmation of an asymptomatic relapse
Especially for diffuse large B-cell lymphoma patients
Benign FDG uptake patterns directly/indirectly associated with chemotherapy
Low level, patchy uptake in residual fibrotic mass and scars
Decreases over months but may persist
Diffuse FDG uptake in all adipose tissue (brown + yellow fat), adrenal/periadrenal regions
Concurrent chemo + protease inhibitors (lymphoma + HIV)
Skeletal muscle uptake
Pattern similar to carbohydrate-insulin effect
Granulocyte colony stimulating factor (G-CSF) or other marrow stimulant drugs
Intense red bone marrow + splenic uptake/enlargement
May persist 2-3 months
Following cessation of chemotherapy
Increased thymic uptake (thymic rebound); diffuse FDG activity in thymus usually 4-8 months following chemotherapy
DIFFERENTIAL DIAGNOSIS
Normal Structures
Thymus, salivary glands, muscle, tonsils
Look for symmetry
Can be asymmetrical if contralateral normal structure paralyzed (vocal cord) or surgically removed (muscle, glands)
Correlate with anatomical findings for confirmation of structure
Reactive Lymph Node Hyperplasia
Typically ↑ FDG uptake
Numerous sites, often symmetrical
LN mildly to moderately enlarged
May resolve over weeks to months
When equivocal, short term follow-up exam is usually helpful
Granulomatous Disease
FDG uptake moderate to marked
Stable over time
Often symmetrical hilar/mediastinal LN
Sarcoid
Lung nodules: Sarcoid “galaxy” sign; multitude of tiny clustered lung nodules along bronchovascular bundle
Garland triad (1-2-3 sign): Symmetrically enlarged bilateral hilar & right paratracheal LN
Enlarged anterior mediastinal LN favors lymphoma
Viral Infections; Infectious Mononucleosis
Minimally enlarged LN
Sub-cm lung nodules that usually resolve completely
Histoplasmosis
Sub-cm lung nodules that often calcify (granuloma)
Calcified normal-sized mildly FDG-avid mediastinal & hilar LN, calcified splenic & hepatic granulomas
Tuberculosis (TB)
Enlarged LN & ipsilateral consolidation in primary TB
Lung nodules may calcify, apical scarring, positive PPD
Cavitary lung lesion in the posterior segment right upper lobe or superior segment of the lower lobes
Cat-Scratch Fever
Enlarged, painful LN
Symptoms resolve over weeks
Whipple Disease
Enlarged abdominal LN with low attenuation center
HIV, AIDS
Higher Grade Lymphomas on Therapy
May show ↓ FDG uptake, mimicking low grade lymphoma
Other Malignancy
FDG PET may identify best candidate site for biopsy
Thoracic, Extrathoracic Malignancy
Enlarged FDG-avid LN accompanied by multiple FDG-avid lung nodules that increase in size and number over time
Small Cell Lung Carcinoma
Markedly enlarged, prominently FDG-avid hilar and mediastinal LN
PATHOLOGY
General Features
General path comments
Most common subtype in adults is diffuse large B-cell lymphoma
Most aggressive subtype with 60% of patients having disseminated disease at diagnosis
More aggressive types
Diffuse large B-cell
Mantle cell
Peripheral T-cell
Fast-growing types
Burkitt lymphoma
Lymphoblastic
Slow-growing types
Marginal zone
Small cell (= CLL when principally in lymph nodes)
Lymphoplasmacytic
Follicular
Extranodal marginal zone lymphomas
GI tract, lung, salivary gland, conjunctiva, and thyroid
Diagnosed as separate entity termed MALT
Etiology
Unknown in most patients
MALT lymphoma (stomach) associated with H. pylori infection
Lymphoplasmacytic lymphoma: 1/3 associated with hepatitis C infection
Hashimoto thyroiditis associated with primary thyroid lymphoma
Epidemiology
NHL incidence ↑ 75% in the past 20 years, with ↑ mortality
Primary NHL of thyroid unusual, only 3.4% of primary thyroid malignancies
Malignant lymphomas as a group compose 5th most frequently occurring type of cancer in the USA
Most common NHL subtypes
Diffuse large B-cell lymphoma, 31%
Follicular lymphoma, 22%
Small lymphocytic lymphoma, 16%
Mantle cell lymphoma, 6%
Peripheral T-cell lymphoma, 2%
Anaplastic large T-cell/null cell lymphoma, 2%
Burkitt-like lymphoma, 2%
Marginal zone nodal-type lymphoma, 1%
Lymphoplasmacytic lymphoma, 1%
Burkitt lymphoma, < 1%
Microscopic Features
T- or B-cell clonality: No Reed-Sternberg cells
Fine-needle aspiration is diagnostic in NHL
FL: Aggressiveness proportionate to percentage of large cells
Staging, Grading, or Classification Criteria
Histologic grading more important than staging of anatomic sites in NHL
Clinical staging
Includes history & physical examination
Imaging of the chest, abdomen, & pelvis
Blood count, chemistry, and bone marrow biopsy
Pathologic staging
Staging laparotomy & pathologic staging no longer routinely performed
REAL/WHO classification
Revised European-American Classification of Lymphoid Neoplasms (REAL), adopted by World Health Organization (WHO)
Distinct disease entities defined by combination of morphology, immunophenotype & genetic features, and distinct clinical features
Relative importance of features varies by disease; no “gold standard”
Includes all lymphoid neoplasms: HD, NHL, lymphoid leukemias, & plasma cell neoplasms
Lymphomas & lymphoid leukemias included because solid + circulating phases are present in many lymphoid neoplasms
REAL/WHO classification of B-cell neoplasms
Precursor B-cell neoplasm: Precursor B-lymphoblastic leukemia/lymphoma (precursor B-cell acute lymphoblastic leukemia)
Mature (peripheral) B-cell neoplasms
B-cell CLL/SLL; B-cell prolymphocytic leukemia
Lymphoplasmacytic lymphoma; splenic marginal zone B-cell lymphoma
Hairy cell leukemia; plasma cell myeloma/plasmacytoma
Marginal zone B-cell/MALT lymphoma
Nodal marginal zone B-cell lymphoma; follicular lymphoma; mantle cell lymphoma
Diffuse large B-cell lymphoma; Burkitt lymphoma/Burkitt cell leukemia
REAL/WHO classification of T-cell and natural killer (NK)-cell neoplasms
Account for 10-15% of NHL
Precursor T-cell neoplasm: Precursor T-lymphoblastic lymphoma/leukemia (precursor T-cell ALL)
Mature (peripheral) T-/NK-cell neoplasms
T-cell prolymphocytic leukemia; T-cell granular lymphocytic leukemia; aggressive NK-cell leukemia
Adult T-cell lymphoma/leukemia (HTLV1+); extranodal NK-/T-cell lymphoma, nasal type
Enteropathy-type T-cell lymphoma; hepatosplenic T-cell lymphoma; subcutaneous panniculitis-like T-cell lymphoma
Mycosis fungoides/Sézary syndrome; anaplastic large cell lymphoma, T/null cell, primary cutaneous type
Peripheral T-cell lymphoma, not otherwise characterized; angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma, T/null cell, primary systemic type
Ann Arbor classification
Anatomic extent of disease in HD & NHL
Stage I
Single LN region (I)
Or localized involvement of a single extralymphatic site in the absence of LN involvement (IE) (rare in HD)
Stage II
≥ 2 LN regions, same side of diaphragm (II); or single extralymphatic site with regional LN
± Other LN stations, same side of diaphragm (IIE)
Stage III
LN regions, both sides of diaphragm (III)
May have extralymphatic extension with adjacent LNs (IIIE) or + spleen (IIIS) or both (IIIE, S)
Stage IV
Diffuse or disseminated disease in ≥ 1 extralymphatic organ
± LN; or isolated extralymphatic organ with or without regional LN
Any liver, bone marrow, lung nodules
Presence or absence of systemic symptoms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree