Abstract
Nonimmune hydrops fetalis (NIHF) encompasses all causes of fetal hydrops that are not caused by the passage of maternal antibodies into the fetal compartment. Secondary to the decreasing prevalence of immune hydrops, NIHF now comprises 90% of all hydrops cases. The dilemma for the treating physician is the wide range of possible etiologies resulting in NIHF, and differentiating when a prenatal treatment is possible. This chapter outlines the definition, epidemiology, pathophysiology, most common etiologies, laboratory and ultrasound evaluation, and the clinical management of fetuses affected by this disorder.
Keywords
hydrops, nonimmune, fetal
Introduction
Nonimmune hydrops fetalis (NIHF) was first explained by Potter, who described fetal hydrops in the nonanemic fetuses of Rh-positive women. With the advent of anti-D immunoglobulin and the decreased incidence of immune-mediated hydrops, NIHF now comprises 90% of all fetal hydrops cases. NIHF presents a diagnostic dilemma for the treating physician secondary to the myriad etiologies that result in NIHF. Although overall fetal mortality remains high, the prognosis for fetuses affected by NIHF differs markedly depending on the underlying etiology. It is essential to attempt to identify the etiology to appropriately counsel the patient about the prognosis, to plan possible corrective intervention, and to arrange for delivery in an appropriately-resourced center to maximize postnatal outcomes.
Definition
NIHF is defined as the presence of fetal hydrops in the absence of a maternal immunologic response to a paternally derived red blood cell antigen in the fetus. Fetal hydrops is traditionally defined as the accumulation of fluid in at least two of the following fetal serous compartments; abdomen, pleural cavity, pericardium, and skin. Polyhydramnios and placental thickening are commonly included in the diagnostic criteria.
Prevalence and Epidemiology
The prevalence of NIHF is estimated to be about 3 : 10,000–6 : 10,000 live births, but is higher in early gestation because of the hidden mortality associated with fetal demises and pregnancy terminations. This is evidenced by the reported incidence of NIHF ranging from 13 : 10,000–60 : 10,000 pregnancies in centers specializing in ultrasound (US) diagnosis. There is a wide range of pathologies resulting in NIHF listed in Table 122.1 and discussed in depth subsequently.
| Cardiovascular | Structural
Arrhythmia
Functional
|
| Chromosomal | Turner syndrome (45X), trisomy 21, trisomy 13, trisomy 18, triploidy |
| Hematologic | Hemorrhage
Abnormal hemoglobin production
Decreased red blood cell production
Increased hemolysis
|
| Infectious | Parvovirus, syphilis, cytomegalovirus, toxoplasmosis, adenovirus, varicella, coxsackie virus, herpes simplex virus, respiratory syncytial virus, rubella, trypanosomiasis |
| Thoracic | Congenital pulmonary malformation, bronchopulmonary sequestration, congenital diaphragmatic hernia, hamartoma, teratoma, hydrothorax |
| Tumors | Teratoma (sacral, mediastinal, pharyngeal), hemangioma, lymphangioma, rhabdomyoma, neuroblastoma |
| Twins | Twin transfusion syndrome Twin reversed arterial perfusion sequence Heterokaryotic twins |
| Gastrointestinal | Midgut volvulus, malrotation, duplication, obstruction, meconium peritonitis, atresia (intestinal or biliary), hepatic (cirrhosis, necrosis or fibrosis), tumor, cholestasis |
| Genitourinary | Congenital nephrosis |
| Placenta, Cord | Chorioangioma, umbilical artery aneurysm, umbilical vein thrombosis |
| Skeletal Dysplasia | Thanatophoric dysplasia, osteogenesis imperfecta, achondrogenesis, short-rib polydactyly, thoracic dysplasia |
| Metabolic | Lysosomal Storage Disease
|
The reported mortality from NIHF varies widely, ranging from 50%–98% and most dependent on the underlying etiology, the gestational age at onset, the gestational age at the time of delivery, and the presence of pleural effusions. In general, the earlier the diagnosis of NIHF is made, the worse the prognosis. Euploid fetuses without major structural abnormalities have a better prognosis.
Etiology and Pathophysiology
Although numerous disparate etiologies result in fetal hydrops, the underlying pathophysiology in all NIHF cases is the altered movement of fluid between fetal vascular and interstitial spaces caused by one of several mechanisms:
- •
disruption of lymphatic drainage,
- •
increased venous pressures,
- •
increased capillary permeability,
- •
decreased osmotic pressure.
Cardiac abnormalities are the most common cause of NIHF, accounting for 20% of cases. They can be subdivided into disorders of structure, rhythm, or function. Structural CHD affects 8–9/1000 live births and the presence of a significant lesion in the presence of hydrops is almost universally lethal. The most common structural lesions associated with NIHF are hypoplastic left heart and atrioventricular septal defects, but tricuspid and pulmonary atresia, tetralogy of Fallot, premature closure of the ductus arteriosus or foramen ovale, and cardiac tumors such as rhabdomyomas and teratomas have all been associated with NIHF. Structural cardiac defects result in hydrops either by increasing right atrial pressure or causing volume overload, with the end result of right-sided heart congestion. CHD has a strong correlation with aneuploidy, with over 15% of cases found to be affected.
Cardiac arrhythmias are also associated with NIHF and can be divided in tachyarrhythmias and bradyarrhythmias. The most common tachyarrhythmias are supraventricular tachycardia, atrial flutter, Wolf-Parkinson-White, and ventricular tachycardia. Tachyarrhythmias lead to increased right atrial pressures and hydrops by decreasing ventricular filling times and causing venous congestion. Digoxin has been used successfully for cardioversion in the nonhydropic tachycardic fetus, but the presence of hydrops decreases placental transfer of this medication. Although it has increased maternal side effects, flecainide reaches therapeutic levels in the fetal compartment in the presence of hydrops and is the preferred medication for fetal tachycardia in this setting. Fetal arrhythmias can also result from maternal autoimmune disorders. Tachyarrhythmias with NIHF secondary to maternal Graves’ disease can be treated with propylthiouracil or methimazole. Bradyarrhythmias are commonly associated with structural abnormalities that disrupt the normal cardiac conduction pathways. Similarly, circulating maternal antibodies associated with systemic lupus erythematous and Sjögren syndrome, specifically anti-Ro/SSA and anti-La/SSB antibodies can cross the placenta and damage the Purkinje fibers in the fetal heart leading to complete heart block. Fetuses can tolerate the ventricular escape heart rate that is normally between 70–75 beats/minute, but when the fetal heart rate drops below 50–55 beats/minute congestive heart failure and resulting hydrops are a risk. The development of hydrops before 32 weeks is associated with a poor prognosis as external cardiac pacing is unlikely to be successful in these preterm neonates. Treatment with steroids to prevent the progression of first or second degree heart block to complete heart block and treatment with B-agonists to increase fetal heart rates have not been shown to be effective and should only be undertaken in a research setting. Vascular tumors of the fetus and placenta can also result in cardiac decompensation and NIHF. Large, vascular lesions such as sacrococcygeal tumors and chorioangiomas can compromise cardiac output via the vascular steal phenomenon. The sequestration of fetal blood in these tumors when they are large and vascular may lead to high output cardiac failure and eventually hydrops.
Chromosomal abnormalities affect 7%–45% of NIHF cases and are more prevalent the earlier the hydrops is identified or in the presence of a structural abnormality. The most common abnormalities are Turner syndrome (45X) and trisomy 21, but trisomy 13, trisomy 18, and triploidy have all been associated. The underlying mechanism causing hydrops varies with each chromosomal abnormality. Turner syndrome is classically associated with cystic hygromas and lymphatic malformations, but is also strongly associated with coarctation of the aorta and hypoplastic left heart syndrome. Trisomy 21 is also strongly correlated with cardiac defects, in particular, atrioventricular septal defects. In addition, trisomy 21 affected fetuses also have an increased incidence of lymphatic malformations and congenital leukemia, both of which are associated with an increased risk of developing NIHF even with normal cardiac anatomy. Fetal karyotype is indicated in all cases of NIHF. If not ordered at the time of initial testing, microarray should be requested if the fetal karyotype is normal, as molecular testing will detect an additional 7% of chromosomal abnormalities in NIHF missed by traditional tissue culture analysis.
Hematologic abnormalities will be found in 7%–55% of NIHF cases and include problems with abnormal hemoglobin production, decreased red blood cell production, increased hemolysis, and fetomaternal hemorrhage (acute or chronic), all of which can result in high output cardiac failure and NIHF. There are differences in the prevalence of certain hematologic abnormalities, determined by the ethnic population studied. The most common inherited hemoglobinopathy is alpha thalassemia, which accounts for over 50% of NIHF in patients of Southeast Asian ancestry and approximately 10% in other ethnic groups. Parents can be suspected as carriers of alpha thalassemia when there is a microcytic (mean corpuscular volume <80 fL), hypochromic anemia with normal iron studies. The most severe fetal form of alpha thalassemia is Bart’s hemoglobinopathy in which the fetus lacks all four copies of the genes responsible for alpha globin chain formation. Other causes of NIHF caused by increased red blood cell destruction and fetal anemia include glucose-6-phosphate dehydrogenase deficiency (X-linked), glucose phosphate isomerase deficiency, and pyruvate kinase deficiency. If fetomaternal hemorrhage is suspected, a Kleihauer-Betke test or flow cytometry should be ordered to detect the presence of fetal cells in the maternal circulation. In these cases, when severe enough, fetal blood sampling and intrauterine transfusion can maintain an adequate fetal hemoglobin concentration and prolong the gestation. Regardless of the cause of the suspected anemia, interrogation of the middle cerebral artery peak systolic velocity (MCA-PSV) is predictive of fetal anemia when it exceeds 1.5 multiples of the median with a sensitivity of nearly 100% and a specificity of 88%. An MCA-PSV is therefore indicated in the evaluation of all hydropic fetuses.
Infections cause 4%–15% of NIHF. Parvovirus is the most commonly encountered infection, but syphilis, cytomegalovirus, and toxoplasmosis have all been reported to cause NIHF. Less frequent infectious causes include varicella, coxsackie virus, herpes simplex virus, and respiratory syncytial virus. Infections may result in hydrops via suppression of erythroid precursors, causing myocardial dysfunction, or hepatitis with a resultant hypoproteinemia. The infectious insult from parvovirus is usually transient, but if the anemia and myocarditis is severe enough, fetal transfusion may be needed to bridge the fetus until normal erythropoiesis is reinitiated ( Chapter 167 ).
Complications from monochorionic (MC) twins make up a small percentage of NIHF, but are one of the causes most amenable to prenatal therapy ( Chapter 159 , Chapter 160 , Chapter 162 ). TTS results from the unequal distribution of volume across placental vascular anastomoses that exist between the twins all MC gestations. The end result is the net loss of volume in one fetus (“donor”), and volume overload in the other fetus (“recipient”). This volume overload in the recipient can potentiate congestive heart failure and hydrops as seen in Stage IV TTS. Fetoscopic laser ablation of the anastomotic vessels separates the circulation of the twins and leads to the eventual resolution of the hydrops in the recipient. Dual survival rates of approximately 70% in Stage IV TTS can be expected after laser surgery when performed in experienced centers ( http://childrens.memorialhermann.org/fetal/ttts/outcomes/ ). Twin reversed arterial perfusion (TRAP) sequence is another complication of MC twinning ( Chapter 163 ). An arterioarterial anastomosis between the normal (“pump”) twin and its acardiac sibling keeps the acardiac twin supplied with blood via retrograde flow through the umbilical artery. If the acardiac mass continues to grow, it may cause high output cardiac failure in the pump twin. Thermal occlusion of the acardiac twin’s umbilical vessels by radiofrequency ablation (RFA) or bipolar cautery can reverse the process. Survival rates >80% have been reported for the pump twin after therapy.
Compromise of the fetal thoracic cavity can also cause NIHF. Thoracic masses, fluid collections, or herniation of fetal tissues into the thoracic cavity can all lead to NIHF by increasing thoracic pressure and decreasing venous return and lymphatic flow. The most common thoracic mass is congenital cystic adenomatous malformation (CCAM) ( Chapter 2 ). Congenital diaphragmatic hernias, bronchogenic cysts, and hamartomas are other rare thoracic causes of NIHF. CCAMs can be micro- or macrocystic. Lesions with rapidly enlarging cystic components may be amenable to thoracocentesis or shunting if hydrops develops and solid lesions have been shown to respond to corticosteroid administration. For the rare, nonresponding, rapidly growing microcystic lesion, sclerosing therapy has been reported as a possible treatment option, but with mixed results. Open maternal-fetal surgery with resection of the mass in potentially viable pregnancies remote from delivery is a last resort as outcomes have been suboptimal with survival rates of approximately 50% ( Chapter 118 ). Hydrothorax is the unilateral or bilateral accumulation of fluid in the pleural space ( Chapter 4 ). This may be an isolated primary process or secondary to another underlying disorder. Prenatal primary hydrothorax corresponds to congenital chylothorax that is caused by lymphatic leakage from a malformation in the development of the thoracic duct. Regardless of whether the hydrothorax is a primary or secondary process, rapid accumulation of fluid in the pleural space can precipitate NIHF in a similar mechanism to solid thoracic masses. Mortality rates when NIHF is associated with hydrothorax can approach 100% if left untreated, however, with in utero therapy, specifically pleuroamniotic shunting, survival rates in primary hydrothorax can be expected to be <70%.
Single gene disorders are a heterogeneous group of disorders that also contribute to NIHF. They include, but are not limited to, inborn errors of metabolism, skeletal dysplasias, neurodevelopment disorders, cardiomyopathies, congenital nephrosis, and mitochondrial mutations. Lysosomal storage diseases are the most common inborn errors of metabolism and include mucopolysaccharidoses, Gaucher disease, and Niemann-Pick disease. They lead to hydrops through the development of visceromegaly and the obstruction of venous return, decreased red blood cell production, and the development of hypoproteinemia. Although they constitute a small percentage of total NIHF cases, a recent review of 678 affected gestations demonstrated that lysosomal storage diseases constitute almost 30% of cases previously classified as idiopathic. Skeletal dysplasias causing NIHF include thanatophoric dysplasia, osteogenesis imperfecta, achondrogenesis short-rib polydactyly syndrome, and thoracic dysplasia. The mechanism of how these disorders cause NIHF is unclear, but may be secondary to hepatic enlargement from increased erythropoiesis in an attempt to compensate for the relatively small bone marrow volume. Many of the skeletal dysplasias associated with NIHF result from mutations in the FGFR3 gene. The importance in identifying any of the aforementioned conditions as the causative agent is that they are usually inherited in an autosomal recessive fashion and thus have a 25% recurrence risk. Part of the investigation of NIHF should include storage of fetal amniotic fluid for further evaluation of these rare diseases.
Clinical Presentation
Most pregnant women in the developed world have a midtrimester US for the evaluation of fetal anomalies and this is when the majority of NIHF cases are identified. With improvements in the quality of US equipment, one could argue that NIHF should never be missed during a routine scan. Patients who do not, however, have access to medical care or fetuses with late-presenting pathologies, may present with maternal symptoms suggestive of NIHF. Polyhydramnios can lead to a uterine size greater than dates, preterm contractions, and preterm labor. Mothers of hydropic infants are also at risk of developing Mirror syndrome, also known as Ballantyne syndrome. This is characterized by a preeclampsia-like picture with generalized maternal edema, hypertension, proteinuria, and often pulmonary edema. It differs from preeclampsia in that the hematocrit is low, as opposed to the hemoconcentration seen in preeclampsia, and it is reversible if the NIHF can be treated and resolves. In cases where the fetal condition is not treatable, delivery is recommended.
Imaging Techniques and Findings
Ultrasound.
The diagnosis of NIHF is dependent on identifying any two of the following:
- •
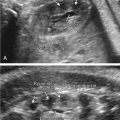
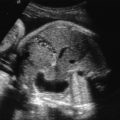
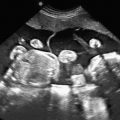
Abdominal ascites: is initially seen as a rim of echolucent fluid on the periphery of the abdominal cavity and as it progresses, the bowel and liver will become more echolucent secondary to the surrounding fluid ( Figs. 122.1 and 122.2 ). Ascites should not be confused with pseudoascites, which is the hypoechoic rim normally seen around the anterior abdominal wall caused by the abdominal muscles. Care must also be taken to differentiate isolated urinary ascites, as seen with a cloacal abnormality or a decompressed lower urinary tract obstruction, from early NIHF.