Lung Transplantation Complications
Lung transplantation can prolong life in a variety of diseases that cause progressive respiratory failure. As of 2015, more than 55,000 adult lung transplantations and almost 4000 heart-lung transplantations had been performed worldwide, more than 3500 a year. The most common indications for lung transplantation in adults have been chronic obstructive pulmonary disease (COPD), pulmonary fibrosis and other interstitial lung diseases (ILDs), and bronchiectasis (most commonly cystic fibrosis); more recently, idiopathic interstitial pneumonias have become the leading cause for lung transplantation. In children cystic fibrosis is the most common disease for which lung transplantation is performed and was the indication in more than 50% of all cases of transplantation in the period 1999 to 2013, and in more than 70% of children older than 6 years. The International Society for Heart and Lung Transplantation maintains guidelines for selection of appropriate candidates; general considerations include a high risk of death within 2 years without lung transplantation and high likelihoods of surviving in the perioperative period and at 5 years after transplantation.
Survival after lung transplantation has significantly increased over the past 20 years. However, transplantation complications still cause significant morbidity and mortality. As of 2013, 1-year survival was 88%, and 5-year survival was 55% for recipients aged 12 years and older. One major center found a 31% rate of hospital readmission in adult lung transplantation within 1 month after discharge; 67% of patients were readmitted within 1 year, usually for pulmonary, infectious, or pleural complications. Chronic complications, such as chronic transplant rejection, also remain a significant cause of long-term mortality.
Surgical Anatomy
Although single-lung transplantation can be performed in selected cases of pulmonary fibrosis, double-lung transplantation is more widely used for cases of COPD and bronchiectasis. Both single- and double-lung transplantation involve harvesting and meticulous hilar dissection of the donor lungs, with sequential creation of bronchial, arterial, and left atrial cuff anastomoses to the recipient. Single-lung transplantations are performed through a lateral thoracotomy, whereas double-lung transplantations use a transverse (clamshell) thoracosternotomy, with an incision extending horizontally across the chest and sternum from axilla to contralateral axilla. Bronchial artery anastomoses are usually not performed, and systemic bronchial revascularization usually takes place over several weeks. As the transient interruption of systemic bronchial arterial supply is thought to be a potential mechanism of bronchial complications, such as stenosis and dehiscence, as well as possibly increasing the rate of the bronchiolitis obliterans syndrome (BOS), a technique of en bloc double-lung transplantation with direct bronchial arterial revascularization has been used by a minority of groups.
Time Course of Complications
The dismal outcomes in the early era of lung transplantation were predominantly due to complications in the first postsurgical month. Airway ischemia with dehiscence was a particular problem before establishment of the sequential double-lung transplantation technique. Although survival rates have markedly improved, significant risks of morbidity and mortality still plague the early postsurgical period. The most common reported causes of death in the first 30 days are noncytomegalovirus (CMV) infections (19%), graft failure (25%), multiorgan failure (12%), cardiovascular causes (12%), and technical failures (11%). The readmission rate for lung transplant recipients within the first month is significantly higher than after treatment for myocardial infarction and complex vascular surgery.
In the intermediate and chronic posttransplantation periods, infections remain an important cause of morbidity and mortality; in addition to bacterial infections, respiratory viruses such as cytomegalovirus and adenovirus, pneumocystis, and fungal infections become more common. Chronic rejection (now called chronic lung allograft dysfunction) is a major concern in the late posttransplantion period and is a leading source of morbidity and mortality after the first year.
Types of Lung Transplantation Complications
Pleural Complications
Prevalence and Clinical Considerations
Pleural complications, such as pneumothorax, hemothorax, chylothorax, and empyema, comprise a large percentage of complications in the first month after transplantation, affecting approximately 22% of patients. Pneumothorax is the most common pleural problem, comprising almost 50% of pleural complications in one study. Pneumothoraces can occur as a result of undersizing of donor lungs, as expansion of the donor lung can take days or weeks to complete. Small air leaks can also be a cause and may spontaneously resolve. Most cases of pneumothorax are managed through watchful waiting or thoracostomy tube placement. The presence of a persistent or increasing pneumothorax despite thoracostomy placement can suggest the presence of a bronchopleural fistula, which can occur in bronchial anastomotic dehiscence.
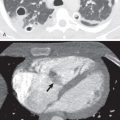
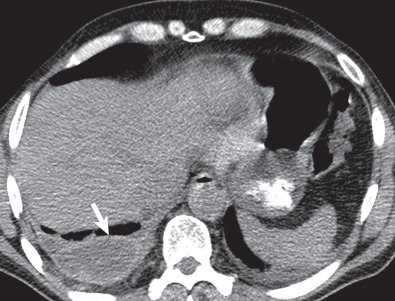
Pleural effusions are common in the first few days of lung transplantation, typically developing in the first hours after transplantation ( Fig. 70.1 ). Effusions are usually exudative, with high red blood cell and neutrophil content, and resolve or decrease in size by day 9 after surgery. An empyema occurred in 27% of patients with pleural fluid in one study; 25% were bacterial in origin, whereas 60% were attributable to fungal organisms, such as Candida albicans . An elevated neutrophil count in pleural fluid of greater than 21% was 70% sensitive and 79% specific for pleural infection in the study. The development of an empyema is associated with an increase in risk of death.
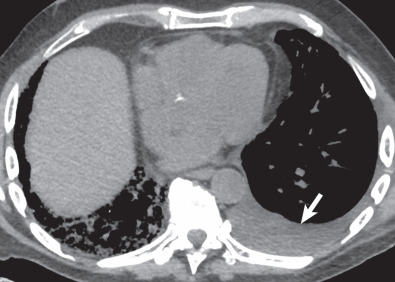
Hemothorax and chylothorax can manifest in the first few hours or days after transplantation, and are uncommon occurrences, usually heralded by a rapid accumulation of pleural fluid or increased thoracostomy tube drainage. Hemothorax may be caused by surgical injury of an intercostal, bronchial, or other artery and can require surgical exploration or embolization. Injury to the thoracic duct can result in chylothorax, for which ligation of the thoracic duct may be required.
Imaging Manifestations
Radiography.
Small pleural effusions are commonly seen at radiography in the first few days after transplantation, but typically begin to decrease in size within the first week. Small pneumothoraces are also very common and usually resolve spontaneously. However, persistent or worsening pleural air or fluid can be crucial to recognition of a significant pleural complication.
Rapid development or sudden increase in size of a postsurgical pleural fluid collection is concerning for the possibility of a hemothorax or chylothorax ( Fig. 70.2 ). Pleural fluid sampling is usually diagnostic, confirming blood or chyle. Persistent or new pleural fluid may suggest empyema; fluid loculation or pleural gas may occur, but are not always present, and may be difficult to discern at portable supine radiography. A new or increasing pneumothorax may suggest a significant air leak or bronchopleural fistula resulting from bronchial dehiscence.
Computed Tomography.
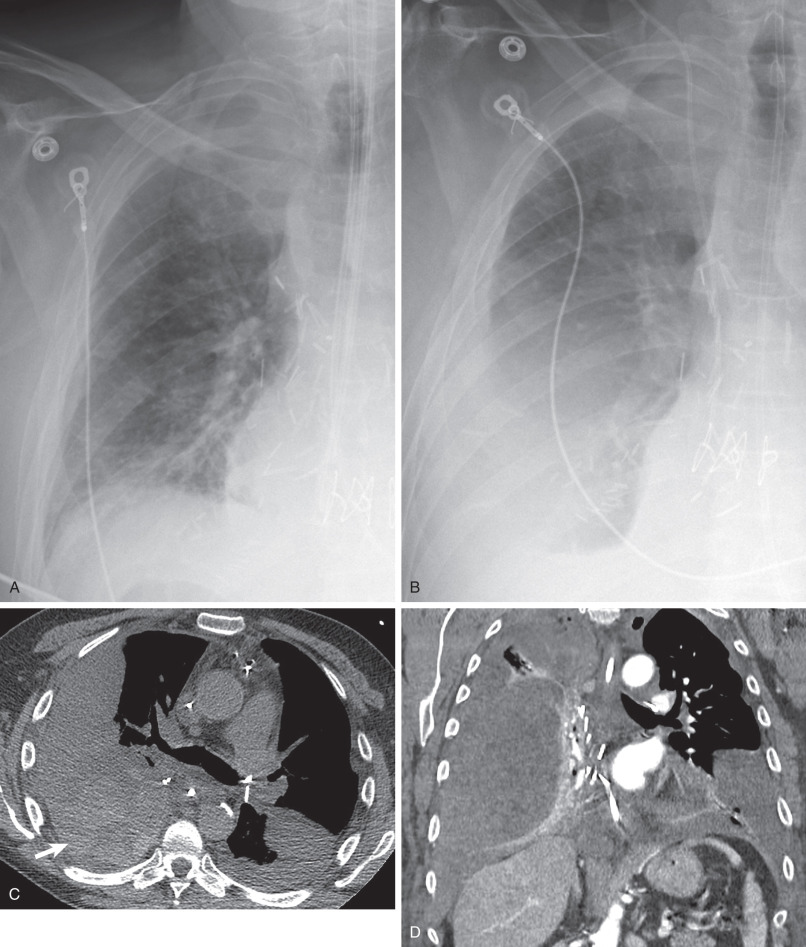
Although not first-line imaging for patients in the immediate postsurgical period, computed tomography (CT) can be used as a supplement to radiography. CT is highly sensitive for the detection of pneumothoraces and pleural fluid collections and can aid in the assessment of fluid loculation ( Fig. 70.3 ; see also Fig. 70.1 ). Detection of high-attenuation pleural fluid confirms hemothorax (see Fig. 70.2 ). The presence of pleural thickening and/or enhancement with the “split pleura sign” can suggest empyema ( Fig. 70.4 ), although these findings can also occur in the chronic setting, resulting from pleuroparenchymal inflammation without active infection.
- •
Pleural complications are common in the first month after lung transplantation.
- •
Pneumothoraces are most common, may be due to a size discrepancy between donor and recipient or air leak, and are usually treated conservatively with thoracostomy tubes and surveillance.
- •
Pleural effusions are common and usually develop within the first hours after transplantation.
- •
Empyemas may occur in almost one-third of patients with pleural fluid and are often bacterial in etiology; loculation and pleural thickening are imaging clues.
- •
A rapidly developing pleural fluid collection in the early postsurgical period suggests the possibility of hemothorax or chylothorax. High-attenuation blood products at CT confirm hemothorax.
Primary Graft Dysfunction
Prevalence and Clinical Considerations
Primary graft dysfunction (PGD) is known by a variety of names, including posttransplantation edema, reperfusion edema, and implantation response. PGD is a form of acute lung injury with noncardiogenic alveolar edema occurring within 72 hours of lung transplantation, representing a complex inflammatory reaction to ischemia/reperfusion injury. Free radicals, cytokines, neutrophils, macrophages, and other immune modulators have been implicated in this condition. PGD is defined and graded based on the presence of bilateral airspace opacities on radiographs, a decreased ratio of partial pressure of oxygen in arterial blood/forced inspiratory oxygen (FiO 2 ), and the absence of alternative causes. Significant PGD is classified as grade 3 (severe) and defined as a ratio less than 200.
Overall, the condition occurs in up to 30% of recipients at some time during the first 72 hours after transplantation. Risk factors for the condition include a history of smoking by the donor, pulmonary hypertension, use of cardiopulmonary bypass, recipient history of sarcoidosis, increased FiO 2 during lung reperfusion, and single-lung transplantation. PGD occurs in as many as 60% of single-lung transplantations, in which about half of patients in one study developed radiographic findings within 3 hours of transplantation.
PGD is associated with a significant increase in 90-day mortality and is associated with a higher incidence of chronic rejection and hospital readmission. All grades of PGD predispose to chronic rejection in the form of bronchiolitis obliterans, resulting in a decrease in long-term survival. PGD may predispose to bronchial complications; in one study recipients with PDG in the first 72 hours after surgery were more than twice as likely to experience bronchial dehiscence, stenosis, bronchomalacia, and hemoptysis, possibly a correlate of direct ischemic/reperfusion bronchial injury. Undersizing of the donor lung may increase the incidence of PGD, possibly through a mismatch in donor and recipient pulmonary arterial sizes that leads to high reperfusion pressures; one study found a 39% decrease in odds of PGD in cases in which oversized lungs were used. Despite several possible medical therapies, supportive care remains the most common treatment.
Imaging Manifestations
Radiography.
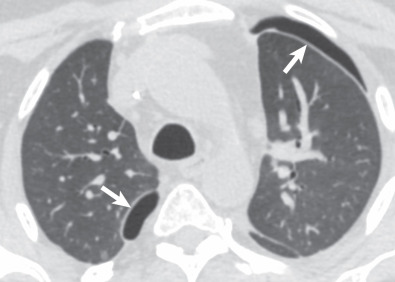
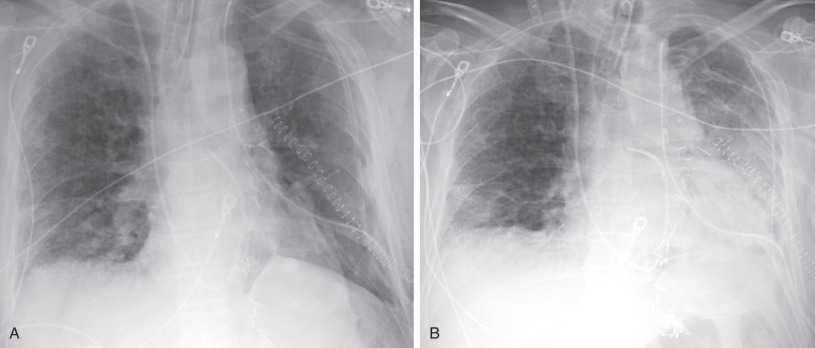
The chest radiograph typically reveals bilateral airspace opacities that usually appear from 24 to 72 hours after transplantation, often with a perihilar and basilar predominance ( Fig. 70.5 ). Some studies of single-lung transplantations have found development of opacities as early as 3 hours after transplantation in approximately 50% of recipients. The opacities may worsen over the course of hours to days but in general show improvement or resolution by postoperative day 5 to 10. The radiographic appearance is nonspecific and can also be seen in cardiogenic edema, pneumonia, hyperacute rejection, and venous obstruction.
Computed Tomography.
CT is generally not typically used for primary evaluation of PGD but usually shows bilateral diffuse ground-glass opacities and/or consolidation. The appearance can be similar to diffuse alveolar damage, cardiogenic edema, and some infections. The opacities improve or resolve within the first week after transplantation.
- •
Primary graft dysfunction (PGD) is an acute lung injury that occurs within 72 hours after transplantation.
- •
PGD occurs in up to 30% of recipients and is associated with both a higher risk of early postsurgical mortality and an increase in incidence of chronic complications, including chronic lung allograft rejection.
- •
Imaging shows diffuse airspace opacities developing 24–72 hours after transplantation, with improvement or resolution after 5–10 days.
- •
Treatment is generally through supportive care.
Rejection
Prevalence and Clinical Considerations
Rejection is classified as hyperacute, acute, or chronic. These conditions have distinctly different times of onset, durations, mechanisms, and outcomes.
Hyperacute rejection is an attack on the donor allograft by preformed recipient antibodies and occurs either during surgery or in the first few postsurgical hours, usually with devastating results and complete graft failure.
Acute rejection is an acute lung injury syndrome that develops over the course of hours or days; although most common in the first year after transplantation, it can occur at any time after transplantation. Symptoms include fever, dyspnea, cough, and hypoxia. Pulmonary function tests (PFTs) can show a decline in forced expiratory volume in 1 second (FEV1) and vital capacity. At histology, acute cellular rejection is characterized by a perivascular monocellular infiltrate. Acute rejection occurs in approximately 35% of recipients within 1 year of transplantation. Although episodes of acute rejection do carry a small risk of mortality, the condition usually responds to steroids or is self-limiting and accounts for less than 4% of all deaths within the first month after transplantation. However, episodes of acute rejection can hasten the onset of or worsen the severity of chronic rejection. Acute rejection can be diagnosed and graded at transbronchial biopsy according to a standard grading system, but a large degree of interobserver variation in scoring of rejection has been noted in a large study.
Acute fibrinous and organizing pneumonia (AFOP) is a form of OP with deposition of fibrin within alveoli and occurs in a small subset of patients after lung transplantation. It may occur in the first year or even first week after transplantation and may mimic acute cellular rejection. Acute and subacute forms have been noted, and the acute form may be mistaken for acute cellular rejection or infection. AFOP may respond rapidly to corticosteroid treatment, but a fulminant acute course may fail to respond to any therapy, with a mortality rate as high as 90%.
Chronic rejection rarely develops before the first few months after transplantation, but is the major cause of long-term and overall mortality in lung transplantation. Chronic rejection causes shortness of breath and progressive respiratory insufficiency. Although previously BOS was traditionally nearly synonymous with the concept of chronic lung transplant rejection, the term chronic lung allograft dysfunction (CLAD) is now widely used in appreciation of subtypes of chronic rejection. CLAD refers to both BOS and the more recently described restrictive allograft syndrome (RAS). RAS carries a poorer prognosis than BOS; BOS patients live on average 3 to 5 years after diagnosis, while those affected by RAS have a mean survival between 6 and 18 months. Some studies have also documented a chronic form of AFOP as a form of CLAD, also with a poorer prognosis than BOS. Definitive medical treatment for CLAD is lacking, and the only effective eventual therapy is retransplantation in appropriate cases. A variety of factors can hasten the development of CLAD, including respiratory viral infections, episodes of acute rejection, and aspiration.
BOS is the most common subtype of chronic rejection, and may affect up to half of patients surviving after the first year of transplantation. The condition shows a progressive pattern of small airway obstruction with air-trapping and chronic respiratory insufficiency. The condition can be diagnosed by a decline in FEV1 greater than 20% below the average of two baseline measurements after lung transplantation. Transbronchial biopsy can also diagnose BOS, but interpretation can be variable. Some studies point to different outcomes based on histology; one study found that histology of true constrictive bronchiolitis was associated with a 3-year survival of 60%, whereas the so-called lymphocytic bronchiolitis subtype survival was 91%.
RAS has been recently appreciated as a subtype of chronic rejection and produces a predominantly restrictive pattern of progressive decline in respiratory function. RAS may account for up to 25% of all CLAD cases. Histopathology shows a pattern similar to idiopathic pleuroparenchymal fibroelastosis, with a peripheral distribution of abnormal collagen deposition, fibroblastic foci, and thickening of the alveolar septal network. RAS has been defined as a decline in FEV1 greater than 20% and a decline in total lung capacity greater than 10% below posttransplantation baseline. The fibrosis in RAS tends to be upper lobe predominant. Although patients with RAS tend to have a worse prognosis than in BOS, subtypes of the condition have been described at CT (described later), and may portend different prognoses, with graft survival in a slow-onset subtype averaging 33.5 months and in a more rapid-onset group averaging 6.4 months.
Imaging Considerations
Radiography.
Portable radiography is the most common imaging modality used in the early posttransplantation period. Both hyperacute and acute rejection usually manifest with diffuse airspace opacities within the transplanted lung(s), often worse in the perihilar regions and lung bases ( Fig. 70.6 ). The radiographic appearance is generally indistinguishable from other causes of airspace opacities, including pulmonary edema, primary graft dysfunction, and infection. AFOP may similarly manifest with bilateral airspace opacities, sometimes with a basilar and peripheral distribution. Distinguishing among these possibilities relies on the time course of development and supportive clinical data. Hyperacute rejection produces progressive opacities that arise within the first few hours of transplantation, whereas PGD usually has onset after 24 hours and begins to improve within 5 days (see Fig. 70.5 ). Acute rejection is more common after the first week and has peak prevalence within the first year.
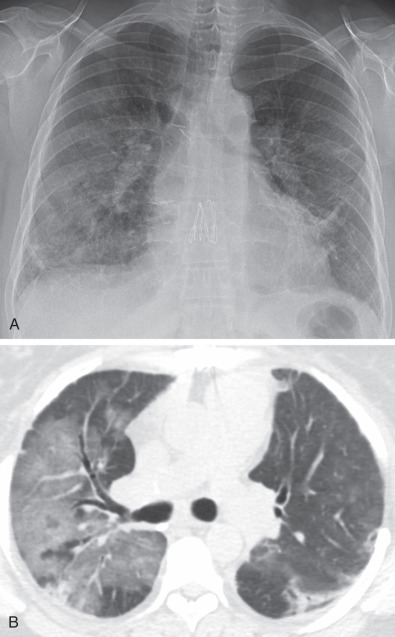
Radiographs have a limited role in the evaluation of chronic rejection and can be normal, especially in cases of BOS. However, hyperinflation, bronchial wall thickening and, at times, bronchiectasis can be seen in BOS, whereas low lung volumes with upper lobe nodularity, consolidation, architectural distortion, and traction bronchiectasis are more typical of RAS ( Fig. 70.7 ).
Computed Tomography.
CT is seldom performed in the setting of hyperacute rejection. Although generally first suspected at radiography, acute rejection typically shows bilateral ground-glass opacities, consolidation, and septal thickening with a basilar predominance. In AFOP CT may show basilar-predominant consolidation, ground-glass opacities, and reticulation.
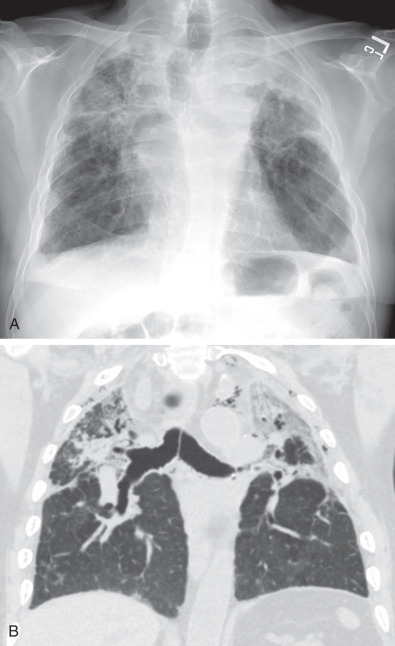
For evaluation of chronic rejection, thin-slice CT is the preferred imaging modality, as it is much more sensitive in detection and characterization of CLAD. A helical series is acquired at full inspiration with an additional axial acquisition performed at maximal expiration to assess for air-trapping. Mosaic attenuation—the presence of alternating low- and high-attenuation areas of lung—is common in BOS ( Fig. 70.8 ). Areas of low-attenuation lung correspond to air-trapping and do not significantly increase in attenuation at expiration. Areas of higher-attenuation lung correspond to more normally aerated lung and increase in attenuation at expiration. A true mosaic attenuation pattern is seen in most cases, with smaller vessel diameters in areas of low attenuation caused by hypoxemic vasoconstriction. However, although these findings can suggest BOS, the degree of mosaic attenuation may not correlate with the BOS stage and degree of obstructive physiology at PFTs. Although mosaic attenuation with air-trapping is often the earliest finding of BOS, helpful secondary findings include bronchial wall thickening and bronchiectasis, which in one study correlated better with airflow limitation than did mosaic attenuation (see Fig. 70.8 ).
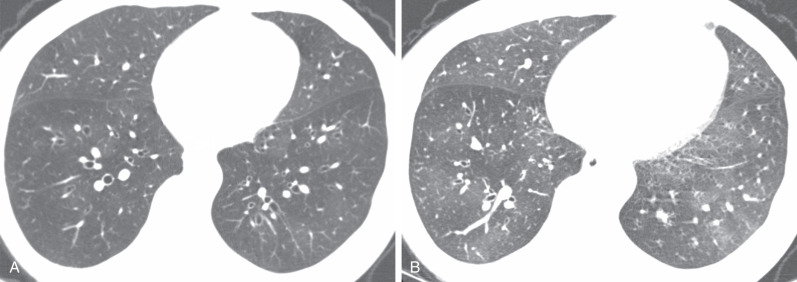
RAS usually shows an upper lobe–predominant pattern of fibrosis at CT that can be identical to idiopathic pleuroparenchymal fibroelastosis. Peripheral reticulation, architectural distortion, traction bronchiectasis, nodules, and consolidation are possible findings (see Fig. 70.7 ). One study proposed two different CT subtypes of RAS; a “mild” subtype gradually developed upper lobe–predominant nodules, reticulation, and volume loss, with a delayed onset of graft failure; the other group showed sudden onset of upper lobe consolidation and volume loss, with a poorer prognosis and median graft survival of only 6.4 months. Three-dimensional CT volumetry reveals a difference in lung volumes among subtypes of CLAD, with recipients with BOS having no significant decrease in lung volumes but those with RAS showing an almost 25% reduction in volume by CT. One study examined the CTs of patients at CLAD onset and found a higher incidence of consolidation and ground-glass opacity in patients who eventually developed RAS, the restrictive phenotype of CLAD, than in those who developed primarily BOS.
- •
Hyperacute rejection is rare and usually manifests during surgery or within the first few hours after transplantation.
- •
Acute rejection is most common in the first year after transplantation but can occur at any time. Mortality is uncommon, but episodes of acute rejection can hasten the development and increase the severity of bronchiolitis obliterans. Diffuse airspace opacities are common.
- •
Acute fibrinous and organizing pneumonia may occur in the first year after transplantation and can mimic acute rejection. Imaging findings include bilateral ground-glass opacities and patchy consolidation.
- •
Chronic lung allograft dysfunction is a major cause of long-term and overall mortality and comprises both bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome.
- •
BOS is the most common subtype of chronic rejection and is a major cause of morbidity and mortality after the first year. A mosaic attenuation pattern with air-trapping, bronchiectasis, and bronchial wall thickening are typical CT features.
- •
Restrictive allograft syndrome is a more recently characterized form of chronic rejection that has restrictive physiology and a worse prognosis than BOS. Imaging features include upper lobe–predominant volume loss, reticulation, architectural distortion, pleural thickening, and traction bronchiectasis.
Airway Complications
Prevalence and Clinical Considerations
The incidence of significant airway complications has been reported as between 2% and 33%. A study of 232 lung transplantations found that longer recipient bronchus length, telescoping anastomotic technique, and prolonged donor ventilation were associated with increased airway complications. Another study of 270 lung transplantation patients found that primary graft dysfunction within 72 hours of transplantation, lung infection in the first month, diabetes, and COPD were independent risk factors for airway complications. Patients may present with unexplained hypoxemia, obstructive PFTs, or atelectasis or postobstructive pneumonia in the involved lobes.
Bronchial dehiscence is an uncommon but potentially devastating complication. In the early perioperative period, dehiscence can arise from ischemia resulting from interruption of the bronchial arterial blood supply during transplantation; other potential contributors include immunosuppressive medications, infection, acute rejection, and donor-recipient bronchial size mismatch. In the intermediate or late period, infection is a more common cause. Fistulas, most commonly to the pleura or esophagus, can rarely occur.
Bronchial stenosis is usually a late complication, occurring more than 3 months after transplantation, usually resulting from granulation tissue, infection, or bronchial ischemia. Aspergillus is a known common offending organism. The most common site of bronchial stenosis is the bronchial anastomosis, but nonanastomotic stenosis can also occur. Although nonanastomotic stenosis can occur in any bronchus, the most common sites are the bronchus intermedius and the right middle lobe bronchus; severe stenosis of the former has been called “vanishing bronchus intermedius syndrome.”
Bronchomalacia can occur at the site of a bronchial stenosis or in isolation. Bronchial collapse at expiration is the key to the diagnosis.
Imaging Considerations
Radiography.
Radiography is used to exclude other sources of respiratory symptoms in lung transplantation patients and is seldom helpful in evaluation of suspected airway complications. In cases in which bronchial dehiscence leads to a bronchopleural fistula, a pneumothorax or pneumomediastinum may occasionally be detected at radiography. Lobar atelectasis can also uncommonly be seen, usually in cases of severe bronchial stenosis.
Computed Tomography.
Thin-slice CT with multiplanar reformats is used for detection and characterization of posttransplantation airway complications. Bronchial dehiscence may be detected as a visible bronchial discontinuity, often with adjacent extraluminal air, pneumothorax, or other evidence of air leak ( Fig. 70.9 ). In one study of CT findings of bronchoscopically proven dehiscence, a bronchial wall discontinuity was 100% sensitive and 94% specific for dehiscence, and an extraluminal air collection was 100% sensitive and 72% specific for the complication. Bronchial stenosis usually manifests as a focal area of bronchial narrowing; bronchial wall thickening or irregularity can occur, and occasionally air-trapping distal to the anastomosis can be seen. Excessive bronchial collapse (>70%) at expiratory series suggests bronchomalacia and can be associated with stenosis or other evidence of bronchial injury.