CHAPTER 88 Noninvasive Imaging of Atherosclerosis
Atherosclerosis is a disease process in which fatty infiltration and inflammation of the wall of medium to large arteries lead to plaque formation and multiple adverse sequelae including rupture, obstruction, and embolism. It is a major source of morbidity and mortality, with 25 million people in the United States demonstrating at least one clinical manifestation of atherosclerosis.1 It has been the number one cause of death in the United States since 1900 except for 1918, the year of the influenza epidemic.2 Atherosclerosis also leads to significant losses in functional capacity and quality of life.
ATHEROSCLEROSIS
Definition
Atherosclerosis is a form of arteriosclerosis characterized by the deposition of plaques containing cholesterol and lipids on the innermost layer of the walls of large and medium-sized arteries.3 The atheroma is a complex of lipids and fibrous tissue with surrounding hypertrophied smooth muscle and inflammatory cells that leads to progressive vessel luminal narrowing and obstruction of blood flow, directly causing or indirectly mediating the many clinical manifestations of atherosclerotic disease.
Prevalence and Epidemiology
The exact overall prevalence of atherosclerosis is not known because of the numerous arterial beds it encompasses and because it is often silent and found only through screening, which is not universally performed. Moreover, atherosclerotic disease often coexists in multiple vascular systems, making the individual disease prevalence not additive. Atherosclerosis does account for almost three quarters of all deaths from cardiovascular disease.2 The majority of these are from coronary atherosclerosis. Although the mortality from coronary heart disease is decreasing, that from noncoronary atherosclerotic disease is increasing, partly owing to the overall aging of the population. Information on the prevalence of noncoronary atherosclerotic disease and the resultant complications is available for the individual arterial systems affected.
Cerebrovascular Atherosclerotic Disease
Cerebrovascular atherosclerosis has the highest impact of noncoronary disease processes. Stroke is the third leading cause of death in the United States, with an annual incidence of 700,000 events. It is associated with high morbidity and mortality, with a 50% 5-year survival and 15% to 30% risk of permanent disability. Sixty percent of new or recurrent strokes are the result of atherothrombotic disease. One third of these are due to carotid atherosclerosis. At the age of 75 years, 53% of women and 63% of men have carotid stenoses of more than 10%.4 The presence of carotid bruits approximately doubles the expected stroke risk, but the disease is often in a different cerebral vascular territory and not related to the initial lesion auscultated. Despite the high morbidity and mortality from stroke, individuals with carotid artery disease are more likely to die of cardiovascular causes than of cerebrovascular disease, underscoring the close relationship between noncoronary atherosclerosis and cardiovascular risk.
Peripheral Atherosclerotic Disease
Defining peripheral arterial disease (PAD) as a noninvasive ankle-brachial index (ABI) below 0.90, its prevalence in the lower extremities is 2.5% for those younger than 60 years and increases markedly with age to 18.8% for those 70 years of age or older.5 Claudication is associated with significant disability and is present in as many as 6% to 7% of the general population 65 years of age and older. The prevalence also varies significantly by risk factor profile. The PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study examined PAD prevalence in the American primary care setting and found that the prevalence was as high as 29% in older Americans or those with significant risk factors (especially tobacco use and diabetes). The disease has high morbidity from both peripheral and cardiovascular events. The annual mortality for those with PAD is 4% to 6% and is as high as 25% for the 1% to 2% of patients with critical limb ischemia.5 Individuals with PAD have a 60% to 80% prevalence of significant coronary artery disease (CAD), with a twofold to sixfold increase in cardiovascular death and a 40% increase in the risk of stroke. PAD is often undetected without careful exercise tolerance questioning and judicious screening, going undiagnosed in as many as 55% of affected individuals.
Aortic Atherosclerotic Disease
Atherosclerosis of the aorta rarely leads to occlusion but causes abdominal aortic aneurysms (AAAs), peripheral embolization of aortic atheromatous material, penetrating aortic ulcers, and intramural hematomas. The prevalences of these entities are difficult to discern as aneurysms have wide variations in definition and the other aortic manifestations are under-reported. The Veterans Affairs Aneurysm Detection and Management (ADAM) study of 125,000 veterans revealed a prevalence of 4.3% and 1.0% for AAAs of 3.0 cm or larger in men and women, respectively, aged 50 to 79 years but only 1.3% and 0.1%, respectively, for AAAs of 4.0 cm or larger.4 The prevalence increases markedly with age; one Scandinavian study showed a peak of 5.9% for men aged 80 to 85 years and 4.5% for women older than 90 years. This disease is almost always asymptomatic because the clinical manifestations are typically catastrophic. The risk of AAA rupture is amplified with increasing diameter; AAAs larger than 6 cm have a 25% yearly risk of rupture.4 Despite the focus on rupture, approximately 60% of patients with AAAs die of other cardiovascular complications.
Renal Atherosclerotic Disease
Renal atherosclerotic disease causes 90% of the cases of renal artery stenosis, an uncommon but highly morbid cause of secondary refractory hypertension and renal failure. The prevalence of renal artery stenosis in the population older than 65 years is 6.8%, but it is much higher in high-risk populations. Patients undergoing cardiac catheterization have a 30% prevalence, whereas renal artery stenosis is present in 22% to 59% of those with PAD.5
Renal arterial disease has a high rate of progression; occlusion occurs in up to 39% of cases by 5 years. Significant renal impairment in individuals with two functional kidneys typically occurs only with bilateral renal artery stenosis, but 44% of patients have bilateral disease. Almost half of individuals with renal artery stenosis have increased creatinine concentration, and 29% have a 25% to 50% decline in glomerular filtration rate. Despite these factors, patients with renal artery stenosis have a 2-year dialysis-free survival of 97.3% and 82.4% for unilateral and bilateral disease, respectively. The risk of cardiovascular events in this population (more than fourfold increase) far exceeds that of significant renal impairment.6 Nevertheless, the mortality rate is high with renal artery stenosis and end-stage renal disease, with a mean life expectancy of only 2.7 years.5
Etiology and Pathophysiology
Response to Injury Hypothesis
The primary step in the formation of atherosclerosis is the development of vessel wall endothelial dysfunction, which occurs as a response to injury from risk factors such as elevations and alterations in low-density lipoprotein (LDL), genetic abnormalities, and toxic free radicals stemming from tobacco use.7
Oxidation Hypothesis
Oxidation of LDL is required for its uptake by macrophages and accumulation within the vessel wall.8 Arterial LDL is progressively oxidized by oxygen free radicals and internalized by macrophages, forming lipoperoxides, a reactive species that triggers further LDL oxidation and plasma membrane destruction. Oxidized LDL is also a potent chemoattractant for macrophages, inducing the expression of vascular cell adhesion molecules and inhibiting macrophage mobility, thereby furthering macrophage and lipid accumulation within the vessel wall. These lipid-laden macrophages are known as foam cells because of their histologic appearance.7 As these foam cells accumulate, they undergo apoptosis and necrosis from increased proteolytic activity, forming a necrotic lipid core.
Progression to Clinical Significance
During the initial stages of atherosclerosis, the blood vessel dilates to maintain lumen size, a process known as the Glagov phenomenon (Fig. 88-1). However, the repeated cycles of inflammation, smooth muscle cell and fibrous tissue proliferation, and expansion of the lipid core eventually overwhelm the compensatory response, leading to progressive luminal obstruction. Decreased luminal blood flow from the increasing vessel blockage will eventually lead to insufficient supply to meet oxygen demand, and ischemia will ensue.
More rapid vessel occlusion can also occur, leading to ischemia and potentially infarction, depending on the vascular bed. The activated T lymphocytes present can secrete matrix metalloproteinases and other lytic molecules that can degrade the fibrous cap, leading to cap rupture and the uncovering of the prothrombotic elements underneath. This exposure, along with other procoagulant factors released by activated inflammatory cells, can induce platelet aggregation and ultimately thrombosis and rapid vessel occlusion (Fig. 88-2).
Risk Factors
The risk factors for atherosclerosis are similar across the multiple arterial beds affected, regardless of the end-organ perfused. They fall into two categories: those that are modifiable and those beyond our control. Modifiable risk factors can be further broken down into those that are predominantly a result of lifestyle indiscretions and those that are primarily manifestations of clinical disease that can be treated (Table 88-1).
TABLE 88-1 Risk Factors for the Development of Atherosclerosis
| Modifiable Risk Factors |
| Lifestyle indiscretions |
| Obesity |
| Tobacco use |
| Physical inactivity |
| Clinical comorbid conditions |
| Lipid abnormalities* |
| Elevated low-density lipoprotein or total cholesterol level |
| Low high-density lipoprotein level |
| Elevated triglyceride levels |
| Diabetes mellitus |
| Metabolic syndrome, insulin resistance |
| Hypertension (both systolic and diastolic are independently associated) |
| Risk Factors (Not Modifiable) |
| Advanced age |
| Male gender |
| Race† |
| Genetic predisposition (positive family history) |
| Prothrombotic and proinflammatory comorbid conditions (such as systemic lupus erythematosus, rheumatoid arthritis) |
| New/Under Investigation |
| Increased lipoprotein(a) |
| High-sensitivity C-reactive protein elevation |
| Homocysteine elevation |
| Increased fibrinogen |
* Each of these lipid abnormalities provides independent incremental risk.
† The black population has a higher rate of atherosclerosis than the white population does.
Risk Factors (Not Modifiable)
Increasing age is the most powerful risk factor for noncoronary atherosclerotic vascular disease (AVD). The atherosclerotic process occurs in a stepwise fashion over time, and those with advanced age are more likely to have a higher burden and greater complexity of disease. Data from the Framingham study show that 7% to 9% of individuals 75 years of age or older have carotid stenoses of 50% or more. In contrast, less than 1% had that degree of obstruction at 50 years of age.4
Gender also plays a significant role in the prevalence of atherosclerosis. However, with the increasing number of female smokers and disproportionate prevalence and rate of increase in obesity, these gender differences are narrowing.2 Race also has a significant impact on the likelihood of atherosclerotic disease. For instance, black populations have a 38% higher incidence than do white populations of ischemic stroke and stroke mortality adjusted for risk factors.4
Known genes that promote lipid abnormalities include apolipoprotein E (APOE) and cholesteryl ester transfer protein (CETP). Mutations in the LDL receptor gene are particularly damaging, leading to familial hypercholesterolemia in its homozygous form and significant lipid abnormalities even when heterozygous, which occurs in approximately 1 in 500 persons.8 Contributors to the inflammatory process include peroxisome proliferator–activated receptor γ, vascular cell adhesion molecule, and tumor necrosis factor α.9 Significant research is necessary to identify new genes and to determine the full impact of known genetic abnormalities, their response to environmental conditions, and the subsequent therapeutic implications.
Modifiable Risk Factors
Many of the known modifiable risk factors have well-established interactions with the pathophysiologic processes of noncoronary atherosclerosis. For example, hypertension causes increased levels of angiotensin II, which stimulates smooth muscle growth and lipoxygenase activity, a contributor to LDL oxidation and inflammation. Lipoxygenase also increases free radical production and subsequently reduces nitric oxide formation. Homocysteine decreases nitric oxide availability in addition to its direct toxicity to the endothelium and its prothrombotic effects.7
Tobacco use and diabetes mellitus appear to confer the greatest risk of noncoronary AVD (Fig. 88-3).9 Tobacco use doubles the risk of ischemic stroke. Smoking also increases the risk of PAD by twofold to sixfold, and more than 80% of those with PAD have smoked or continue to do so. This effect occurs in a dose-dependent manner.5 In the Edinburgh Artery Study, the odds ratio (OR) for PAD with tobacco use (OR, 1.8-5.6) was approximately twofold to threefold higher than for CAD (OR, 1.1-1.6).
The proposed pathophysiologic mechanisms for the increased risk of disease in PAD versus CAD are (1) increased endothelial dysfunction (measured through von Willebrand and tissue plasminogen activator antigens), (2) reduced circulating antioxidants, (3) increased plasma fibrinogen levels, and (4) altered lipoprotein profiles. The Edinburgh Artery Study specifically addressed the differential odds ratios by measuring risk factors and analyzing the prevalence of these two conditions in 1592 subjects both with and without a history of tobacco use.10 This study confirmed increased levels of von Willebrand and tissue plasminogen activator antigens (markers of endothelial disruption), reduced antioxidant levels, and increased fibrinogen levels. However, correction for these variables decreased the PAD odds ratio to only 2.7 from 3.9, with little change in the CAD odds ratio. Although the differential effect of tobacco use was partly mitigated by adjusting for these potential contributors, it is clear that other unknown mechanisms still predominate.10
Diabetes mellitus is the other of the two most significant modifiable risk factors, increasing the risk of PAD by 2- to 4-fold and ischemic stroke by 1.8- to 6-fold.5,11 The risks of critical limb ischemia and major amputation are also higher with diabetes. The pathophysiologic mechanism underlying this increased risk is multifactorial. Increased levels of C-reactive protein promote apoptosis and stimulate procoagulant tissue factors, leukocyte adhesion molecules, and inhibitors of fibrinolysis. The hyperglycemia, insulin resistance, and fatty acid production associated with diabetes reduce the bioavailability of nitric oxide, decreasing vasodilation and allowing increased smooth muscle cell proliferation and platelet activation. Finally, diabetes increases procoagulant tissue factor and fibrinogen production, leading to a hypercoagulable state.12 Unlike tobacco use, diabetes does not appear to increase the risk of noncoronary AVD disproportionately to the risk of CAD.
Unlike with coronary vascular disease, the lipid abnormality most strongly associated with noncoronary AVD is the combination of high triglyceride and low high-density lipoprotein (HDL) levels, which are also highly linked with diabetes. These lipid abnormalities are closely involved in the noncoronary atherosclerotic process along with increased LDL. Triglyceride-rich lipoproteins stimulate smooth muscle cell proliferation and extracellular matrix deposition. Low levels of HDL increase atherosclerotic risk through a relative decrease in its beneficial processes, including reverse cholesterol transport for its excretion, endothelial protection, and anti-inflammatory effects.8
Novel Risk Factors
Greater understanding of the most common risk factors associated with noncoronary AVD have led to the development of risk scores for stroke and claudication based on the Framingham data. However, novel contributors of risk, especially those estimating inflammation, such as high-sensitivity C-reactive protein, lipoprotein(a), and homocysteine, are challenging these existing paradigms.9 Lipoprotein(a), for instance, self-aggregates and increases inflammation by impairing fibrinolysis through regulation of fibrinogen activator inhibitor 1 and by inducing smooth muscle cell proliferation.
There is some variation in risk factors based on the anatomic localization of disease. For instance, in aortic disease, tobacco use continues to play a significant role (partly because of elastin degradation). It and male sex confer the highest risk for AAAs, whereas those of Asian descent rarely develop this disorder. A family history of AAA is especially important. In PAD, however, diabetes plays a larger role, especially for the female gender. PAD appears to have a higher incidence in African-American and Hispanic subgroups. Other than these examples, few data are available on gender- and ethnicity-based risk differences.9
Manifestations of Disease
Clinical Presentation
History
The presenting symptoms of noncoronary AVD in the different arterial beds can be found in Table 88-2, which can also be used as a comprehensive vascular review of systems.
TABLE 88-2 Common Presenting Clinical Findings and Vascular Review of Systems
| Condition | Clinical Symptoms and Review of Systems |
|---|---|
| Cerebrovascular disease | |
| Peripheral arterial disease | |
| Abdominal aortic disease | |
| Renal artery stenosis | |
| Mesenteric arterial disease |
AAA, abdominal aortic aneurysm; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ESR, erythrocyte sedimentation rate.
AAAs are almost always asymptomatic, although they can be picked up on a careful physical examination. An essential part of a general review of systems is a history of aortic aneurysmal disease in a first-degree relative. Up to 28% of patients with an AAA have a first-degree relative with disease, and the relative risk for male relatives of affected men is as high as 18.5 Individuals with a family history may have onset of disease at a younger age, although the rate of progression and location do not appear to differ. Inflammatory AAA is one subset that does often have symptoms with no significant differences in risk factor makeup.
Physical Examination
The key aspects of the peripheral vascular examination include the following5:
Given the limited sensitivity and specificity of the history and physical examination for noncoronary AVD, any concerning findings should be evaluated further through noninvasive vascular testing.5
Imaging Indications and Algorithm
Carotid intimal-medial thickness (CIMT) measurement by ultrasonography, on the other hand, is primarily used to evaluate cardiovascular risk and is typically performed in asymptomatic individuals. It is best used in patients with an intermediate risk of cardiovascular disease or with a strong family history, an especially severe risk factor, or other reason for which the optimal aggressiveness of medical therapy in an individual is unknown. More than 1000 asymptomatic patients in nine studies have shown a strong association between an abnormal CIMT and increased risk of cardiovascular death, nonfatal myocardial infarction (MI), stroke, or a combination of these. The presence of carotid plaque or a CIMT greater than the 75th age- and gender-matched percentile indicates the need for more aggressive medical therapy.13
Noninvasive evaluation of PAD almost always starts with ABI ascertainment as a high-risk asymptomatic screening method or to evaluate symptoms in the absence of arterial insufficiency ulcers or critical limb ischemia (Fig. 88-4). ABIs correlate highly with the site and severity of peripheral arterial obstruction as well as with overall cardiovascular risk. Subsequent further studies typically involve high-resolution MRA, computed tomographic angiography (CTA), or digital subtraction angiography.

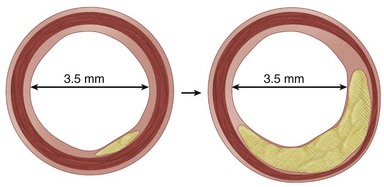
 FIGURE 88-1
FIGURE 88-1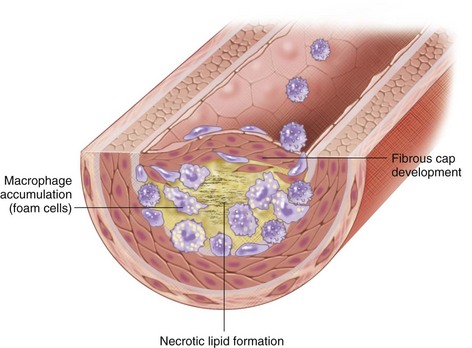
 FIGURE 88-2
FIGURE 88-2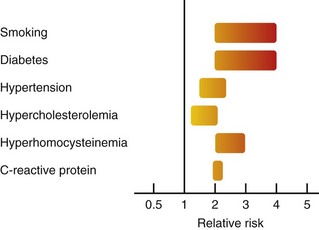
 FIGURE 88-3
FIGURE 88-3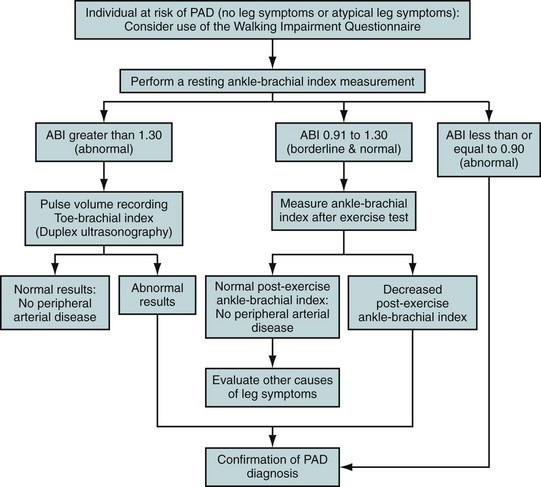
 FIGURE 88-4
FIGURE 88-4

