Fat Embolism
Etiology, Prevalence, and Epidemiology
The term fat embolism refers to the presence of globules of free fat within the pulmonary vasculature. Fat embolism is common among trauma patients, especially those with long bone or pelvic fractures. In the context of trauma, fat embolism is also common after extensive injury to subcutaneous fat, such as occurs in severe beatings or liposuction. Fat embolism must be differentiated from fat embolism syndrome, which is defined by the presence of clinical signs and symptoms resulting from fat emboli. The clinical manifestations of fat embolism syndrome consist of the triad of hypoxia (95%), confusion (60%), and petechial rash (33%). Unfortunately, the classic triad is rarely present, and the clinical manifestations of fat embolism syndrome range from an indolent course to fulminant respiratory failure. The incidence of clinically significant disease in patients who have simple tibial or femoral fractures is generally believed to be about 1% to 3%. In individuals who have more severe trauma, the incidence of clinically evident embolism is probably in the range of 10% to 20%. Less common causes of fat embolism include severe beatings (embolism of subcutaneous fat), orthopedic procedures (e.g., intramedullary prosthesis insertion, arthroplasty), liposuction, severe pancreatitis, sickle cell disease, hematopoietic stem cell (bone marrow) transplantation and harvesting, and venous hyperalimentation.
Clinical Presentation
The symptoms of fat embolism syndrome usually appear gradually; dyspnea, neurologic symptoms, fever, and petechial rash typically develop 12 to 36 hours after injury. The delay in the development of clinical symptoms is presumably due to the time required for hydrolysis of neutral fat to toxic free fatty acids. Cough, hemoptysis, and pleuritic chest pain occur occasionally. Signs include pyrexia, tachypnea, and tachycardia. Acute cor pulmonale with cardiac failure, cyanosis, and circulatory shock may occur.
Symptoms of systemic fat embolism are seen in up to 85% of patients who have pulmonary disease. The symptoms are mainly related to the central nervous system and include confusion, restlessness, stupor, delirium, seizures, and coma. A petechial rash often develops 2 to 3 days after embolization, particularly along the anterior axillary folds and in the conjunctiva and retina.
Pathophysiology
Two pathogenetic mechanisms have been implicated in the development of pulmonary abnormalities after fat embolism. The first is mechanical vascular obstruction, predominantly by fat globules themselves and possibly enhanced in some cases by platelet or red blood cell aggregates. A second potential mechanism involves conversion of neutral triglycerides, the form in which fat appears to be transported to the lungs, into free fatty acids by endothelial lipases. On histologic examination, the presence of fat within pulmonary arterioles and capillaries can be suspected on hematoxylin and eosin–stained sections when there are round to oval spaces, 20 to 40 µm in diameter, apparently compressing red blood cells to one side. Definitive histologic diagnosis requires the use of fat-soluble dyes on unfixed (frozen) tissue or other special techniques.
Manifestations of the Disease
Radiography
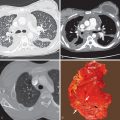
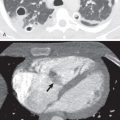
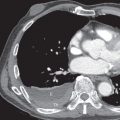
The radiographic findings may be subtle and consist of bilateral hazy areas of increased opacity (ground-glass opacities) or patchy consolidation ( Fig. 52.1 ). In more severe cases, widespread consolidation resembles the radiographic appearance of acute respiratory distress syndrome from any cause. In one review of 22 patients with fat embolism syndrome, 2 had normal chest radiographs throughout hospitalization, and 20 developed pulmonary abnormalities. In all cases the radiographic findings consisted of bilateral areas of consolidation consistent with diffuse pulmonary edema. The time to appearance of evident radiographic lung injury was within 24 hours of initial trauma in 50% of cases, between 24 and 48 hours in 20%, and more than 48 hours in 30%. Of the patients with abnormal radiographs, 10 of 20 (50%) had complete resolution of the edema pattern within 1 week of development of opacities and 30% within 1 to 4 weeks; 20% died without resolution of the radiographic findings. Pleural effusions are typically absent.

Computed Tomography
The computed tomography (CT) findings of fat embolism syndrome include bilateral patchy or diffuse ground-glass opacities, patchy or confluent areas of consolidation, and poorly defined centrilobular nodules measuring less than 10 mm in diameter ( Fig. 52.2 ; see also Fig. 52.1 ). CT may demonstrate parenchymal abnormalities in patients with normal chest radiographs. In an investigation of 9 patients with fat embolism syndrome who had normal radiographs, all were found to have abnormalities on high-resolution CT; 7 had ground-glass opacities, and 2 had small nodular opacities. A CT review of 18 patients with fat embolism syndrome demonstrated consolidation with a dependent distribution (see Fig. 52.2 ) and patchy or peripheral predominant ground-glass opacities in the majority of patients. There was often lobular sparing with sharp margination between areas of involved and noninvolved lung that is thought to be secondary to variations in perfusion at time of embolization (see Fig. 52.1 ). Less common manifestations included lobular ground-glass opacities or consolidation, smooth septal thickening, bronchial wall thickening, and a crazy paving pattern. The degree and extent of consolidation and ground-glass opacities correlate well with disease severity. Centrilobular and subpleural nodules are usually seen in association with ground-glass opacities but may be the predominant or only abnormality evident on high-resolution CT in patients with fat embolism. The nodular opacities tend to be located in the centrilobular regions, along the interlobular septa, and along the interlobar fissures. Tree-in-bud opacities are uncommon but have been reported. Intraarterial fat is usually not seen, but the pulmonary trunk is often dilated (size >29 mm). Rarely, there are reports of patients developing pulmonary fibrosis months after fat embolization.

Differential Diagnosis
The time lapse between trauma and radiographic signs of fat embolism is usually 12 to 36 hours. This delay differentiates fat embolism from traumatic lung contusion, in which the radiographic opacity invariably appears immediately after injury. In addition, although the lung contusion usually clears rapidly (in about 24 hours), resolution of fat embolism generally takes 7 to 10 days and occasionally as long as 4 weeks. Further differentiation lies in the extent of lung involvement; contusion seldom affects both lungs diffusely and symmetrically.
Several tests have been evaluated to identify fat embolism, but none of these is specific for the diagnosis. Thrombocytopenia is frequently present and may be associated with disseminated intravascular coagulation (DIC). Hypocalcemia may develop because of the affinity of calcium ions for free fatty acids released by the hydrolysis of embolized fat. Lipiduria is relatively common, and hematuria and proteinuria are seen occasionally.
The diagnosis can be difficult to make, partly because of the relative nonspecificity of signs and symptoms and partly because clinical abnormalities may be related more directly to the cause of the emboli (e.g., trauma-associated shock). Some investigators have advocated the use of bronchoalveolar lavage and analysis of harvested macrophages for the presence of fat. However, patients who do not have fat embolism syndrome may also have lipid-laden macrophages in their bronchoalveolar lavage fluid, and the definitive diagnostic threshold is unclear; some investigators propose a value as low as 5% and others a value as high as 30%.
Synopsis of Treatment Options
There is no specific treatment available for fat embolism syndrome. The mainstay of treatment is therefore supportive. Prophylactic corticosteroids (methylprednisolone) and heparin may have beneficial effects, but their utility is controversial. The outcome in patients who receive supportive care is generally favorable; the mortality is less than 10%.
- •
Common causes are severe trauma and long bone and pelvic fractures.
- •
Typical symptoms include dyspnea, confusion, and petechial rash, which develop 12 to 36 hours after injury.
- •
Radiographic findings consist of bilateral ground-glass opacities or consolidation.
- •
CT findings include patchy or diffuse ground-glass opacities, patchy or confluent areas of consolidation, lobular sparing, and poorly defined centrilobular nodules.
- •
As distinct from lung contusion, radiographic findings of fat embolism are not present immediately after the injury.
Amniotic Fluid Embolism
Etiology, Prevalence, and Epidemiology
Amniotic fluid embolism is a rare but severe complication of pregnancy. In a population-based cohort of 3 million hospital deliveries in Canada between 1991 and 2002, the total rate of amniotic fluid embolism was 14.8 per 100,000 multiple-birth deliveries and 6.0 per 100,000 singleton deliveries. Of the 180 cases of amniotic fluid embolism in women with singleton deliveries during the study period, 24 (13%) were fatal. Medical induction of labor nearly doubled the overall cases of amniotic fluid embolism, and the association was stronger for fatal cases. Maternal age of 35 years or older, cesarean or instrumented vaginal delivery, polyhydramnios, cervical laceration or uterine rupture, placenta previa or abruption, eclampsia, and fetal distress were also associated with an increased risk.
Clinical Presentation
The majority of patients are in the 35th to 42nd week of pregnancy at the time of embolization. The clinical manifestations are typically abrupt, with sudden onset of cardiovascular collapse, cyanosis, and hemorrhage or DIC. In less severe cases the initial manifestation is progressive dyspnea. Although these abnormalities begin during spontaneous labor in most patients, they occur after delivery in about 30% (10% spontaneous and 20% after cesarean section). The majority of patients experience seizures.
Pathophysiology
It is likely that little if any amniotic fluid enters the maternal circulation during normal labor and that significant embolism occurs only when there is disruption of the uterine wall in association with rupture of the placental membranes. Such disruption can occur at several sites, the most common probably being the endocervix or lower uterine segment. Traumatic tears in the small veins in these regions can occur during normal labor but are of no significance if they are covered by fetal membranes; however, if such veins have separated from the fetal membranes, uterine contractions can “pump” amniotic fluid into the maternal venous circulation. Amniotic fluid can also enter the maternal circulation at the placental site, usually in cases of uterine rupture, placenta previa, or cesarean section when the incision involves the site.
The pathophysiologic consequences of intravascular amniotic fluid are complex and probably related to several processes. These include pulmonary vascular obstruction by amniotic fluid particulates, such as meconium; left ventricular dysfunction, possibly as a result of ischemia secondary to pulmonary hypertension and right ventricular dysfunction; pulmonary edema, related to the left ventricular dysfunction just described or to damage to the vascular endothelium by some constituent of amniotic fluid; and an immunologic reaction pathogenetically similar to anaphylaxis. The main histologic abnormality is the presence of squames, mucin, and bile derived from meconium within small pulmonary vessels.
Manifestations of the Disease
Radiography and Computed Tomography
The principal radiographic finding is bilateral airspace consolidation with or without pleural effusions, which is indistinguishable from acute pulmonary edema of other causes ( Fig. 52.3 ). Whether cardiac enlargement accompanies the edema depends on the severity of pulmonary hypertension and consequent cor pulmonale with or without left ventricular failure. The consolidation may persist or resolve within a few days.

Differential Diagnosis
The diagnosis of amniotic fluid embolism is based on the rapid development of a complex constellation of findings with sudden cardiovascular collapse: acute left ventricular failure with pulmonary edema, DIC, and neurologic impairment. Because the predominant radiographic manifestation is widespread airspace consolidation, the main differential diagnoses are massive pulmonary hemorrhage and aspiration of liquid gastric contents. The diagnosis of amniotic fluid embolism should be considered, particularly in patients with risk factors such as medical induction of labor and placenta previa or abruption; it is supported by identification of squames, mucin, or hair fragments in samples of pulmonary capillary blood aspirated through a pulmonary arterial catheter.
Synopsis of Treatment Options
There are no specific pharmacologic or other therapies that prevent or treat the amniotic fluid embolism syndrome. The treatment is therefore supportive and involves aggressive management of multiple types of shock simultaneously. The mortality rate of clinically recognized cases remains high at 19% in a recent study and up to 61% in older cohort studies; 25% to 50% of patients die within the first hour of the disease and most of the rest within 12 hours. Serious neurologic sequelae are common in survivors.
- •
Amniotic fluid embolism occurs in 14.8 per 100,000 multiple-birth deliveries and 6.0 per 100,000 singleton deliveries and carries a substantial mortality rate.
- •
Main risk factors are multiple-birth deliveries, medical induction of labor, cervical laceration, uterine rupture, placenta previa or abruption, and eclampsia.
- •
Clinical presentation: dyspnea, cyanosis, sudden onset of cardiovascular collapse, hemorrhage, or disseminated intravascular coagulopathy. Symptoms usually begin during labor and before giving birth.
- •
Radiologic manifestation: bilateral airspace consolidation that resembles pulmonary edema.
Tumor Embolism
Etiology, Prevalence, and Epidemiology
Hematogenous pulmonary metastases are derived from tumor fragments lodged within pulmonary vessels. In most cases these tumor fragments are small and therefore do not result in clinically or radiologically apparent vascular obstruction. However, when tumor emboli are of sufficient size or number, the clinical, pathologic, and radiographic manifestations can be identical to those of pulmonary thromboembolism. The manifestations can include pulmonary infarction, acute cor pulmonale and sudden death, and a slowly progressive syndrome of dyspnea and pulmonary hypertension. Tumor embolism is seen most commonly in metastatic renal cell carcinoma, hepatocellular carcinoma, carcinoma of the breast, carcinoma of the stomach, and carcinoma of the prostate. Tumor embolism may be more frequent after interventions that increase the fragmentation of the tumor mass, such as surgery, radiation therapy, or chemotherapy.
Manifestations of the Disease
Radiography
Intravascular tumor emboli are seldom recognized on the chest radiograph.
Computed Tomography and Nuclear Medicine
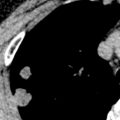
Tumor emboli may be seen as filling defects in the central pulmonary arteries ( Fig. 52.4 ), as nodular or beaded thickening of the peripheral pulmonary arteries, or as nodular and branching centrilobular opacities (tree-in-bud pattern) representing enlarged centrilobular arteries ( Fig. 52.5 ). In contrast to thromboembolic disease, tumor macroembolism is resistant to fibrinolytics and anticoagulation therapy. Occasionally, tumor microembolism and lymphangitic carcinomatosis may manifest on nuclear medicine perfusion scintigraphy with contour mapping of the pulmonary segments, whereby numerous small perfusion defects outline the bronchopulmonary segments (see Fig. 52.5 ).
- •
Common primaries: renal cell carcinoma; hepatocellular carcinoma; carcinomas of the breast, stomach, and prostate
- •
Seldom evident on the chest radiograph
- •
Findings on CT:
- •
Intravascular filling defects
- •
Nodular or beaded thickening of the peripheral pulmonary arteries
- •
Nodular and branching centrilobular opacities (tree-in-bud pattern)
- •


Air Embolism
Etiology, Prevalence, and Epidemiology
Air emboli may have their origin in either the greater or the lesser circulation. In venous air embolism the air enters the systemic venous circulation and passes to the right side of the heart and then to the lungs; clinical and functional manifestations are therefore related to obstruction of the pulmonary circulation and affect predominantly the lungs. In systemic (arterial) air embolism air typically enters the pulmonary venous circulation and passes to the left side of the heart and then to the systemic arteries; the effects are therefore manifested mainly in the heart, the spinal cord, and the brain. Venous air embolism is a frequent iatrogenic complication, occurring most commonly with surgery, insertion and maintenance of intravenous (IV) devices, diagnostic or therapeutic air injection (e.g., arthrography), and barotrauma caused by positive-pressure ventilation. Venous air embolism has been reported to occur in more than half of all cesarean sections. Small quantities of air can be found in the central veins in up to 23% of patients during contrast material administration for CT. Noniatrogenic air embolism sometimes occurs in scuba divers as a result of gas bubble formation in the blood, which is due to rapid reduction in the ambient pressure during a diver’s ascent.
Systemic air embolism occurs when the wall of a vessel exposed to air is disrupted and the pressure of the air exceeds the pressure in the vessel. The most common cause is probably penetrating thoracic trauma, either iatrogenic or accidental. Other causes include open-heart surgery (residual air in pulmonary veins released into the circulation after cross-clamping is terminated), transthoracic needle biopsy, and thoracentesis. Embolism can also occur in several situations in which the thorax is intact. One of the most common is scuba diving, in which the pathogenesis may be related to poor ventilation of a bulla or cyst because of partial or complete obstruction of its feeding airway. Identical barotrauma of ascent can occur in airplane passengers. In both these situations, tissue disruption is related to the increase in air pressure in an enclosed space as a result of a decrease in ambient pressure. Systemic air embolization can also occur in a variety of situations in which there is underlying lung disease, such as severe asthma, and during assisted positive-pressure ventilation.
Clinical Presentation
The majority of patients with pulmonary air embolism are asymptomatic. Clinical symptoms include dyspnea and lightheadedness; chest pain occurs occasionally. Physical findings include tachycardia, tachypnea, and systemic hypotension. Signs of pulmonary edema may also be evident. Migration of air into systemic vessels supplying the brain or heart may result in convulsions, coma, or chest pain.
Pathophysiology
The effects of embolized air in the pulmonary circulation depend on its quantity and rate of entry. Rapid injection of a large amount of air may result in the formation of an air block in the outflow tract of the right ventricle that prevents pulmonary arterial blood flow. Smaller amounts, infused slowly, appear to exert an effect at the level of the distal pulmonary arteries and arterioles. Some of this effect is probably related to vascular obstruction by air bubbles themselves; however, reflex vasoconstriction and the formation of fibrin emboli as blood and air are whipped together in the right-sided heart chambers may also be important. The overall effect of these processes is a transient increase in pulmonary vascular resistance and arterial pressure. Both clinical and experimental observations indicate that pulmonary edema complicates some cases of pulmonary air embolism, probably as a result of increased microvascular permeability.
Another cause of pulmonary air embolism is related to the air that reaches the pulmonary vasculature as part of the decompression syndrome. When a diver spends a prolonged period breathing gas at greater than atmospheric pressure, excess air dissolves in the blood and tissue fluids. With too rapid an ascent and return of partial pressures to lower values, air comes out of solution and forms small bubbles that can be carried in the systemic veins to the right side of the heart and pulmonary vasculature. Oxygen coming out of solution can be disposed of easily by metabolic consumption, but the inert nitrogen is much slower to be cleared. The bubbles cause lung microvascular damage and noncardiac pulmonary edema. This form of pulmonary decompression sickness, which has been called the chokes, is a relatively uncommon form of decompression illness that is sometimes fatal.
Manifestations of the Disease
Radiography
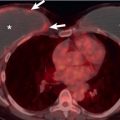
The principal sign of air embolism is the presence of gas in cardiac chambers or pulmonary or systemic vessels. In pulmonary air embolism the gas is present in the right-sided heart chambers and central pulmonary arteries; in systemic air embolism it can be identified in the left-sided heart chambers, aorta, or more peripheral branches of the systemic arterial tree, such as the neck, shoulder girdles, or upper part of the abdomen. Other manifestations of pulmonary air embolism include pulmonary edema, focal oligemia, enlarged central pulmonary arteries, and atelectasis. The radiographic findings associated with air embolism in a study of 31 scuba divers included pneumomediastinum ( n = 8), subcutaneous air ( n = 3), pneumopericardium ( n = 2), and pneumothorax and pneumoperitoneum ( n = 1 each).
Computed Tomography
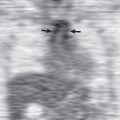
Pulmonary air embolism is seen particularly well on CT ( Fig. 52.6 ). In a study of 100 patients who received IV contrast material that was injected by hand and followed by a drip infusion, asymptomatic venous air embolism was documented in 23. The most common site was the pulmonary trunk. In another series of 677 patients who underwent contrast-enhanced CT, air emboli were detected in 79 (12%). They were located in the pulmonary trunk in 54 (8%), the superior vena cava in 12 (1.8%), the right ventricle in 10 (1.5%), the subclavian or brachiocephalic vein in 6 (0.9%), and the right atrium in 5 (0.7%). Seven patients (1%) had emboli at more than one site. Systemic air embolism is uncommon and is most commonly seen after penetrating or iatrogenic trauma. It manifests as air within the left-sided heart chambers, aorta, or systemic arteries ( Fig. 52.7 ).