Nontraumatic (or spontaneous) intracranial hemorrhage most commonly involves the brain parenchyma and subarachnoid space. This entity accounts for at least 10% of strokes and is a leading cause of death and disability in adults. Important causes of spontaneous intracranial hemorrhage include hypertension, cerebral amyloid angiopathy, aneurysms, vascular malformations, and hemorrhagic infarcts (both venous and arterial). Imaging findings in common and less common causes of spontaneous intracranial hemorrhage are reviewed.
Spontaneous or nontraumatic intracranial hemorrhage (ICH) accounts for approximately 10% to 15% of strokes in the United States. The hemorrhages are typically parenchymal, but may also primarily involve the subarachnoid space, subdural space, intraventricular space, or, rarely, epidural space. The clinical presentation of a parenchymal hemorrhage is usually with the sudden onset of a focal neurologic deficit, often accompanied by headache, alteration in the level of consciousness, seizure, and/or nausea and vomiting; intracranial extracerebral hemorrhage more typically presents with headache and alteration in the level of consciousness, although focal neurologic deficits may also be present, notably as a consequence of tissue shift and brain herniation.
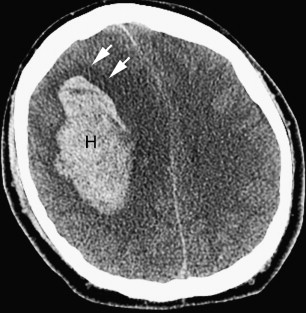
The clinical presentations of ischemic stroke and ICH may be similar, and neuroimaging is indicated for further evaluation of the type of stroke and likely cause. Noncontrast computed tomography (CT) of the head is generally the first study performed on presentation to medical attention, because CT is rapid, widely available, and safe. CT is also highly sensitive to acute ICH, which appears dense compared with normal brain tissue and cerebrospinal fluid (CSF) ( Fig. 1 ). Many institutions also perform CT angiography (CTA) and, less commonly, CT perfusion (CTP) as part of the assessment of acute ICH. MR with gradient-recalled echo (GRE) imaging is sensitive to acute hemorrhage, but MR (often with MR angiography [MRA], MR venography [MRV], and/or MR perfusion) is usually performed following CT to assess the cause of a known hemorrhage and to evaluate its effect on the rest of the brain parenchyma. In the setting of acute ischemic stroke with hemorrhagic transformation, MR with diffusion-weighted (DW) imaging is often key to making a diagnosis that could be missed on a noncontrast CT alone, and even on a CT that includes CTA and CTP, depending on the site and size of the infarct that underlies the hemorrhage. In the setting of spontaneous ICH, MR is generally most useful in assessing the cause of hemorrhage rather than the presence of hemorrhage itself; in addition, MR is sensitive to both acute and chronic hemorrhage, whereas CT is most sensitive to acute blood products.
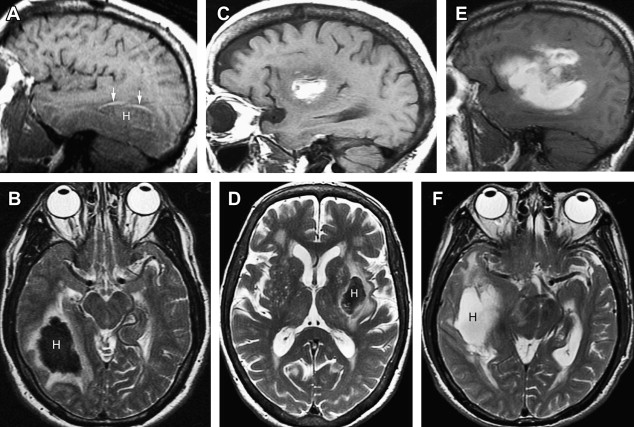
The appearance of blood products is more complex on MR than on CT. On CT, acute blood is dense compared with brain tissue and CSF, and gradually decreases in density over days to weeks depending on the size of the hematoma. On MR, the appearance of the hematoma changes on T1- and T2-weighted images as the blood products evolve from oxyhemoglobin to eventually ferritin and hemosiderin. The evolution of blood products on MR sequences is shown in Fig. 2 and summarized in Table 1 .
| Timing | Blood Product | T1W Image | T2W Image |
|---|---|---|---|
| Hyperacute (<12 h) | Intracellular oxyhemoglobin | Isointense to hypointense | Hyperintense (variable hypointense rim) |
| Acute (hours to days) | Intracellular deoxyhemoglobin | Isointense | Hypointense |
| Subacute-early (few days) | Intracellular methemoglobin | Hyperintense | Hypointense |
| Subacute-late (week to months) | Extracellular methemoglobin | Hyperintense | Hyperintense |
| Chronic-early | Extracellular methemoglobin; ferritin/hemosiderin wall | Hyperintense | Hyperintense with low signal rim |
| Chronic-late | Hemosiderin | Isointense | Hypointense |
The causes of nontraumatic ICH are numerous. This review focuses on the entities most commonly associated with nontraumatic ICH, such as hypertension and cerebral amyloid angiopathy (CAA), but some increasingly recognized less common entities such as isolated cortical vein thrombosis, moyamoya disease, and the reversible cerebral vasoconstrictive syndrome are also discussed.
Hypertensive hemorrhage
Long-standing hypertension is the leading cause of spontaneous intraparenchymal hemorrhage in adults. Pathophysiologically, long-standing hypertension leads to reactive hyperplasia of smooth muscle cells in cerebral arterioles. Eventually, smooth muscle death occurs, and vascular walls are replaced by collagen. Vascular occlusion or ectasia may then result, with either ischemic or hemorrhagic consequences. Hypertensive hemorrhages may also arise at sites of previous ischemic damage to the walls of small arteries and arterioles.
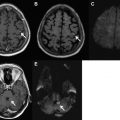
Hypertensive hemorrhages tend to occur in the basal ganglia, thalami, cerebellum, and pons; the lobar white matter may also be affected ( Fig. 3 ). A homogeneous, round or ovoid hematoma in one of these locations in an older adult patient has a high likelihood of being a result of the microangiopathy associated with long-standing uncontrolled hypertension. The age of the patient (whether older than 45 years or not), the presence of a history of hypertension, and the presence of certain associated imaging findings may help determine the yield of further imaging to assess for causes other than hypertension. On a CT scan, one may see evidence of microvascular ischemic change in the white matter as well as evidence of lacunar infarcts; these changes are common in the setting of chronic hypertension and increase the likelihood that hypertension is the underlying cause of the acute hemorrhage. On MR, one may also see evidence of white matter microvascular changes on fluid attenuated inversion recovery (FLAIR) and T2-weighted images, as well as evidence of prior lacunar infarcts or remote parenchymal hemorrhages. It is important to include a T2*-weighted sequence in the MR imaging protocol, because the presence of microbleeds in the brainstem and deep gray nuclei supports the likelihood of chronic hypertension and a hypertension-related hemorrhage ( Fig. 4 ). Brain hemorrhages related to drug abuse (typically cocaine and methamphetamine) may mimic the appearance of brain hemorrhages associated with long-standing hypertension on imaging studies, and likely share a common pathophysiology, but they tend to occur in a younger age group.
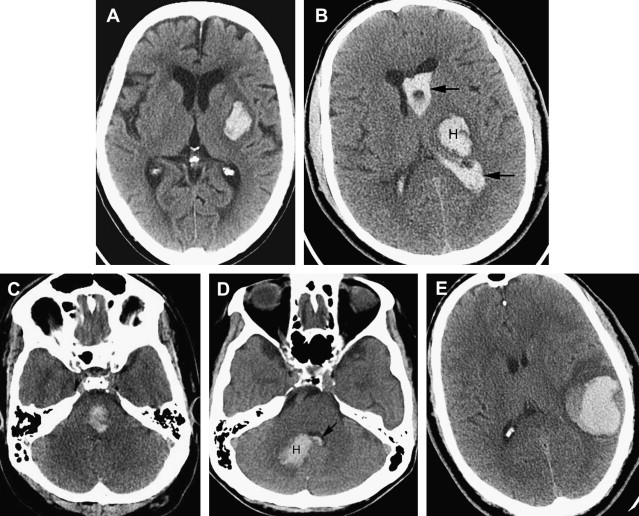
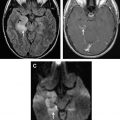
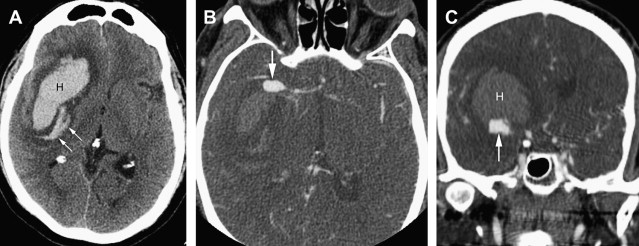
Intraparenchymal hematomas may expand by more than one-third of their original volume in up to 38% of patients who are imaged early. Hematoma expansion most commonly occurs in the first few hours after symptom onset and has been associated with poor outcome. Risk factors for hematoma growth include time to initial CT scan (in that the earlier a patient is scanned after ICH onset, the more likely it is that the next CT will show ICH expansion), larger ICH volume on the initial CT, and oral anticoagulant use. Other potentially important factors include hyperglycemia, renal failure, low serum cholesterol, platelet dysfunction, and persistently increased blood pressure. On CTA and on the contrast-enhanced CT obtained after the CTA, the presence of focal contrast extravasation is associated with hematoma expansion, providing potential justification for consideration of performing these studies on presentation to the hospital. Performing CTA can also help avoid the pitfall of misinterpreting hemorrhages caused by underlying pathologic conditions such as aneurysms or vascular malformations as hypertensive in patients who are older and who do have a diagnosis of hypertension ( Fig. 5 ).
Supportive care (including blood pressure control and correction of coagulopathy) is the mainstay of management of acute intraparenchymal hemorrhage, with surgical evacuation having a more limited role in its management. Most experts agree that cerebellar hematomas that are greater than 3 cm in diameter should be emergently evacuated if the patient is deteriorating neurologically or if the hematoma exerts mass effect on the brainstem or causes hydrocephalus. Furthermore, based on the results of the International Surgical Trial in Intracerebral Hemorrhage (STICH), surgical evacuation may be considered for patients with superficial (<1 cm from the cortical surface) lobar hematomas. Whether minimally invasive surgical techniques (using mechanical devices and/or endoscopy) to remove the hematoma offer benefit over conventional craniotomy/craniectomy is unknown and currently under investigation.
Cerebral amyloid angiopathy
CAA, a disorder that primarily affects individuals older than 60 years, is defined by the accumulation of amyloid in the walls of small and medium-sized cerebral arteries within the brain and leptomeninges. Both inherited and sporadic forms of CAA exist; the inherited form is less common, has an earlier onset of symptoms, and is more strongly associated with dementia. Amyloid deposition in cerebral blood vessels can have several clinical consequences. First, it may be asymptomatic, as it is known that more than 50% of individuals older than 80 years have pathologic evidence of CAA. Second, it may weaken the vessel wall, leading to a rupture and ICH. Third, it can obliterate the vascular lumen, leading to ischemia and contributing to leukoencephalopathy.
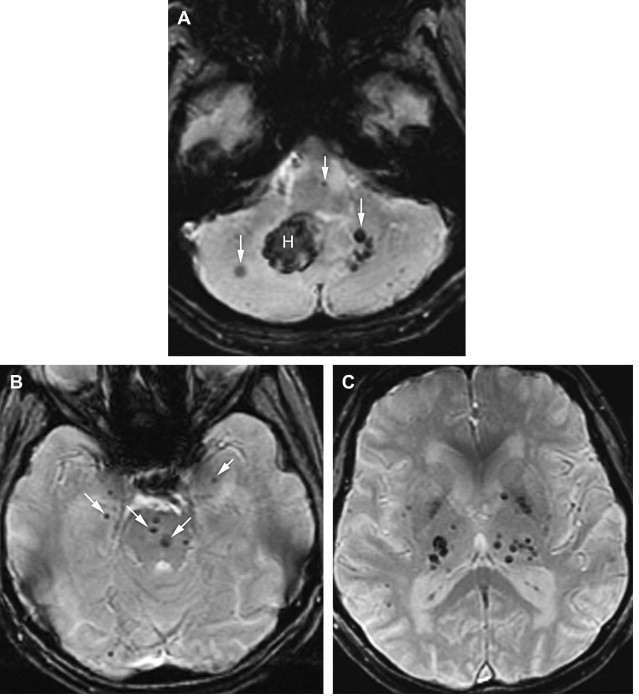
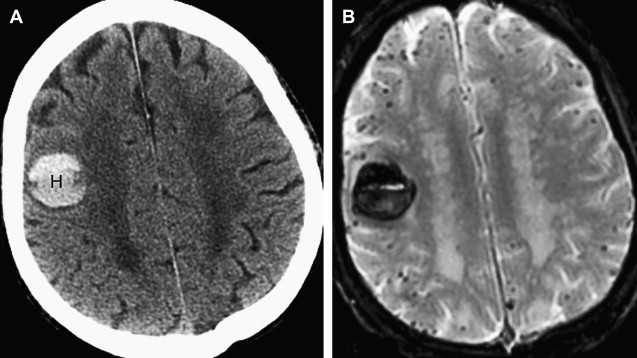
CAA-related hemorrhages are said to account for 5% to 20% of nontraumatic cerebral hemorrhages in elderly patients, so it is second only to hypertension as a cause of spontaneous ICH. The hallmark of the CAA-related hemorrhage is a lobar, cortical, or cortical-subcortical hemorrhage affecting normotensive individuals older than 55 years; the hemorrhages are frequently multiple and recurrent, and often extend to the subarachnoid space. An important clue to the diagnosis of CAA is the presence of multiple petechial hemorrhages (microbleeds) in characteristic locations identified on T2*-weighted MR images ( Fig. 6 ). Unlike the microbleeds associated with chronic hypertension, which are typically located in the brainstem and deep gray nuclei, the microbleeds of CAA tend to be located peripherally in the cerebellum and in the cortical-subcortical region of the cerebral hemispheres. Newer MR imaging methods such as susceptibility-weighted imaging are exquisitely sensitive to chronic blood products and will likely increase the frequency of diagnosis of CAA and other hemorrhagic disorders in coming years, as will the increasing prevalence of high-field (3 T and greater) imaging systems.
The use of warfarin in patients with CAA is controversial. Warfarin use is associated with an annual ICH risk of 0.3% to 0.6%, and risk factors for warfarin-associated ICH include older age, leukoaraiosis, and CAA. Given the associated stroke risk of 5% to 12% per year with atrial fibrillation and 4% per year in patients with mechanical valves, most clinicians choose to place patients with atrial fibrillation or mechanical heart valves on chronic warfarin therapy despite imaging evidence of an increased risk of warfarin-associated ICH in patients with CAA. In patients who have suffered a warfarin-associated ICH, the risk of subsequent cardioembolic complications needs to be weighed against the risk of ICH recurrence.
Cerebral amyloid angiopathy
CAA, a disorder that primarily affects individuals older than 60 years, is defined by the accumulation of amyloid in the walls of small and medium-sized cerebral arteries within the brain and leptomeninges. Both inherited and sporadic forms of CAA exist; the inherited form is less common, has an earlier onset of symptoms, and is more strongly associated with dementia. Amyloid deposition in cerebral blood vessels can have several clinical consequences. First, it may be asymptomatic, as it is known that more than 50% of individuals older than 80 years have pathologic evidence of CAA. Second, it may weaken the vessel wall, leading to a rupture and ICH. Third, it can obliterate the vascular lumen, leading to ischemia and contributing to leukoencephalopathy.
CAA-related hemorrhages are said to account for 5% to 20% of nontraumatic cerebral hemorrhages in elderly patients, so it is second only to hypertension as a cause of spontaneous ICH. The hallmark of the CAA-related hemorrhage is a lobar, cortical, or cortical-subcortical hemorrhage affecting normotensive individuals older than 55 years; the hemorrhages are frequently multiple and recurrent, and often extend to the subarachnoid space. An important clue to the diagnosis of CAA is the presence of multiple petechial hemorrhages (microbleeds) in characteristic locations identified on T2*-weighted MR images ( Fig. 6 ). Unlike the microbleeds associated with chronic hypertension, which are typically located in the brainstem and deep gray nuclei, the microbleeds of CAA tend to be located peripherally in the cerebellum and in the cortical-subcortical region of the cerebral hemispheres. Newer MR imaging methods such as susceptibility-weighted imaging are exquisitely sensitive to chronic blood products and will likely increase the frequency of diagnosis of CAA and other hemorrhagic disorders in coming years, as will the increasing prevalence of high-field (3 T and greater) imaging systems.
The use of warfarin in patients with CAA is controversial. Warfarin use is associated with an annual ICH risk of 0.3% to 0.6%, and risk factors for warfarin-associated ICH include older age, leukoaraiosis, and CAA. Given the associated stroke risk of 5% to 12% per year with atrial fibrillation and 4% per year in patients with mechanical valves, most clinicians choose to place patients with atrial fibrillation or mechanical heart valves on chronic warfarin therapy despite imaging evidence of an increased risk of warfarin-associated ICH in patients with CAA. In patients who have suffered a warfarin-associated ICH, the risk of subsequent cardioembolic complications needs to be weighed against the risk of ICH recurrence.
Hemorrhagic transformation of ischemic stroke
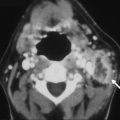
Hemorrhagic transformation of an ischemic stroke (HTIS) can be misdiagnosed as a primary intraparenchymal hemorrhage if the underlying ischemic infarct is not appreciated from clinical or imaging evaluation, but in most cases it is readily recognized as a complication of prior arterial infarction ( Fig. 7 ). HTIS has been associated with baseline stroke severity, hyperglycemia, uncontrolled hypertension, advanced age, use of thrombolytics, nonrecanalization, and use of anticoagulants, among other risk factors. HTIS may be evident on imaging studies alone (usually petechial hemorrhage), or may cause deterioration of a patient’s clinical condition (parenchymal hematoma). HTIS is reported as complicating from 2% to 40% of ischemic strokes, depending how carefully one looks for it with various imaging modalities. Symptomatic intracerebral hemorrhage in the setting of ischemic infarction treated with intravenous or intraarterial thrombolytic therapy occurs in only approximately 6% to 12% of patients.
Predicting which ischemic strokes may undergo hemorrhagic transformation is of interest as use of thrombolytic agents becomes more widespread and as these agents are more frequently administered in later time windows (eg, more than 4.5 hours after symptom onset). Radiographic risk factors for hemorrhagic transformation after thrombolytic therapy include large areas of hypoattenuated brain parenchyma and a hyperdense middle cerebral artery (MCA) sign on the pretreatment head CT. In addition, patients who do not recanalize are at higher risk for subsequent hemorrhage. On CTP, the permeability-surface area product has been suggested to be useful in identifying patients with acute ischemic stroke who are likely to develop hemorrhagic transformation. On MR, a malignant pattern of large regions of DW and perfusion-weighted imaging abnormality combined with early reperfusion seemed to predict symptomatic hemorrhagic transformation and poor clinical outcome. In addition, the cause of ischemic stroke influences whether hemorrhagic transformation occurs. Septic emboli, especially those of fungal cause, are often accompanied by parenchymal hemorrhage, and this diagnosis should be strongly considered in the appropriate clinical setting when a patient presents with multifocal ischemic infarcts associated with hemorrhage ( Fig. 8 ). Ischemic stroke is discussed in greater detail in the article Imaging of Ischemic Stroke by Leiva-Salinas and Wintermark elsewhere in this issue.
Aneurysmal subarachnoid hemorrhage
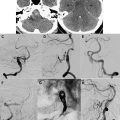
The most common cause of subarachnoid hemorrhage (SAH) is trauma, but aneurysmal SAH accounts for at least 85% of nontraumatic SAH cases. Aneurysmal SAH accounts for only 5% of stroke cases, but with an associated mortality of 12% to 66% in various studies, this stroke subtype accounts for the most life-years lost to stroke. Common risk factors for aneurysmal SAH are hypertension and smoking, and it affects women more frequently than men. Patients with aneurysmal SAH typically present with acute onset of severe headache, often described as the worst headache of the patient’s life. Other symptoms and signs may include vomiting, seizures, nuchal rigidity, cranial nerve palsies, and alteration in the level of consciousness ranging from confusion and drowsiness to coma. The diagnosis of acute SAH is made on noncontrast CT, or by lumbar puncture when the CT scan is negative and clinical suspicion is high. The likelihood of missing acute SAH on the initial CT scan increases as the time interval between symptom onset and time of the CT scan increases. Once nontraumatic SAH has been diagnosed, the patient usually undergoes an urgent evaluation focused on identifying the cause of the SAH: saccular aneurysms are the most common cause, but dissecting intracranial aneurysms and vascular malformations (discussed in detail later) may also present with spontaneous SAH.
On nonenhanced CT (NECT) imaging, aneurysmal SAH typically presents as increased density in the basal cisterns and/or Sylvian fissures. The pattern of hemorrhage may provide insight into the likely location of the aneurysm (ie, blood in the anterior interhemispheric fissure is associated with rupture of an anterior communicating artery aneurysm). Some patients may have an intraparenchymal hematoma in addition to blood in the subarachnoid space, and the hematoma is typically adjacent to the dome of the aneurysm. In some cases the offending aneurysm may be large enough to be identified as a mass on an NECT, or it may be seen as a relative filling defect within the dense cisternal blood; calcified aneurysms can also be identified on NECT. The most common locations for saccular aneurysms include the anterior communicating artery, posterior communicating artery, and MCA trifurcation, but many other locations (basilar tip, posterior inferior cerebellar artery, anterior choroidal artery) may also be seen.
In the past, urgent catheter angiography was generally indicated for assessment of nontraumatic SAH. In the past decade, however, CTA has evolved as the initial study of choice in many centers ( Fig. 9 ). On CTA, the typical saccular aneurysm is seen as a rounded contrast-filled outpouching of the vessel wall. It is important to scrutinize both the source images and the three-dimensional (3D) reformations of the CTA carefully, because in some cases one or the other of these image sets may show the aneurysm to better advantage.