, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Hypoplastic left heart syndrome (HLHS) is characterized by hypoplasia of the left heart and varying degrees of hypoplasia or atresia of the aortic valve and ascending aorta. It is included in the spectrum of conditions that can result in a cardiac circulation with a functionally single ventricle. Left untreated, HLHS is invariably lethal. Advances in palliative surgical procedures (Norwood procedure, bidirectional cavopulmonary shunt, modified Fontan procedure) have increased the survival rate in children with HLHS.
31.1 Palliative Treatment of Hypoplastic Left Heart Syndrome
Hypoplastic left heart syndrome (HLHS) is characterized by hypoplasia of the left heart and varying degrees of hypoplasia or atresia of the aortic valve and ascending aorta. It is included in the spectrum of conditions that can result in a cardiac circulation with a functionally single ventricle. Left untreated, HLHS is invariably lethal. Advances in palliative surgical procedures (Norwood procedure, bidirectional cavopulmonary shunt, modified Fontan procedure) have increased the survival rate in children with HLHS.
In the 1980s, Norwood et al. described a two-stage palliative surgical procedure for the treatment of HLHS [1]. Later, this approach was modified to the currently used three-stage reconstruction [2]. The surgery is done in stages to protect the pulmonary circulation and to avoid right ventricular volume overload.
Stage I of the modified Norwood procedure is performed within the first few days of life. It isolates the hypoplastic left ventricle from the circulation and converts the right ventricle into the main ventricle pumping blood to the lungs and the body (univentricular physiology). The main pulmonary artery and the aorta with its associated coronary arteries are connected using a side-to-side anastomosis, creating a neoaorta (Figs. 31.1 and 31.2). The atrial septum is excised, creating a nonrestrictive ASD or common atrium to facilitate systemic circulation. Pulmonary flow is established via a modified Blalock–Taussig shunt or more recently a right ventricle-to-pulmonary artery conduit (Sano procedure) (Figs. 31.3 and 31.4) [3]. The disadvantage of the Blalock–Taussig shunt is that coronary artery blood flow which occurs during both diastole and systole causes a coronary “steal phenomenon” (coronary blood flow is diverted to the low-pressure pulmonary circulation) leading to myocardial ischemia and ventricular dysfunction [4–7]. The Sano procedure eliminated the diastolic runoff of coronary blood flow thus avoiding the coronary steal phenomenon [8–10]. The complications of the Sano procedure, however, are conduit stenosis and right ventricular aneurysm formation [11, 12].
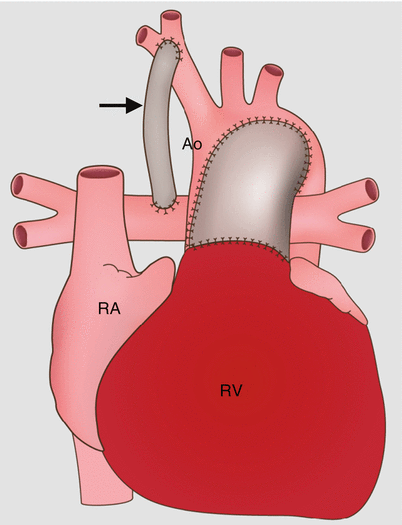
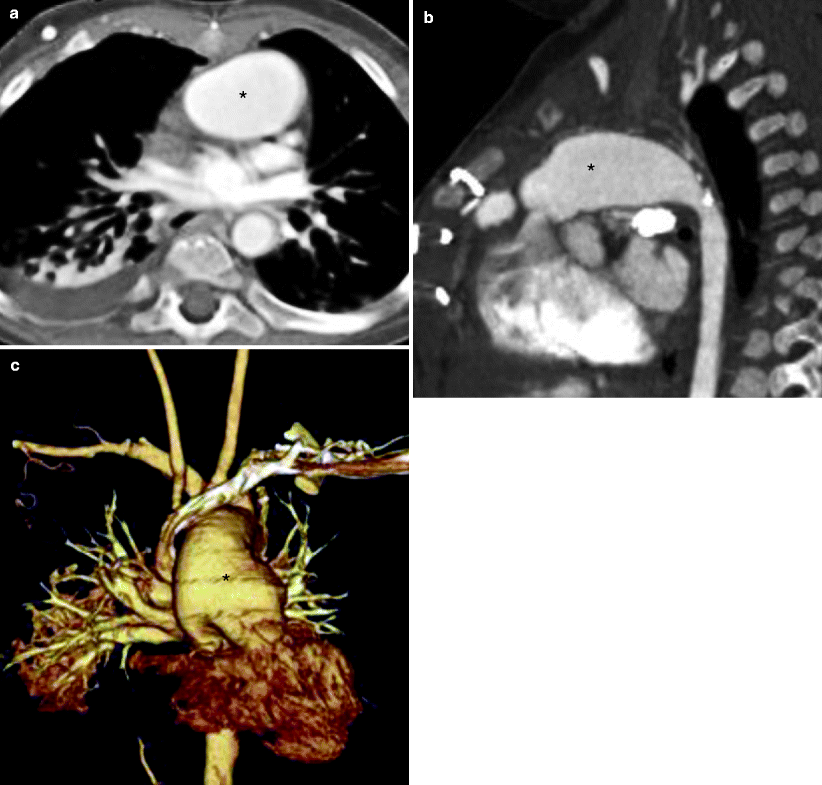
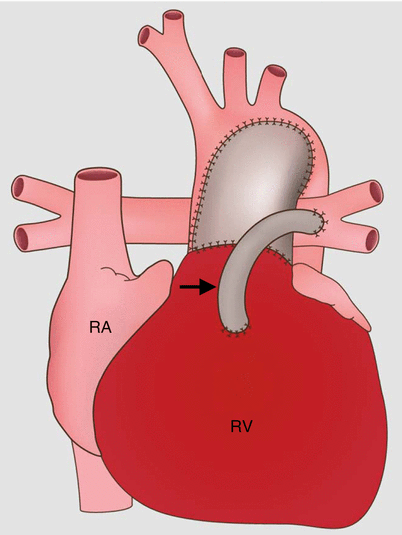
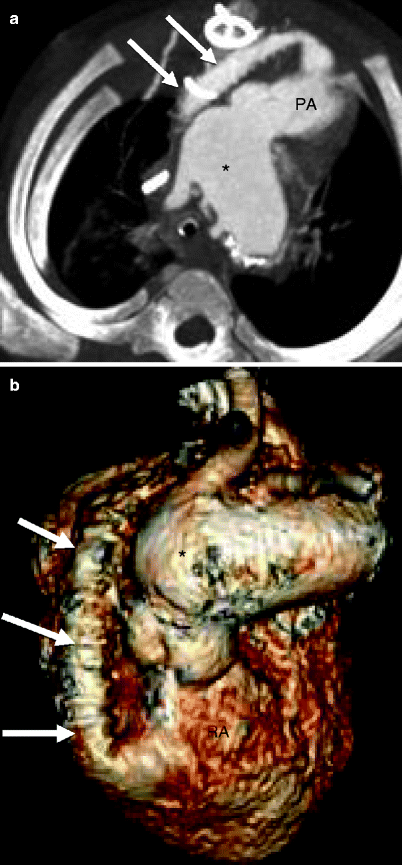

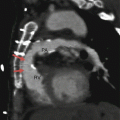
Fig. 31.1
Stage 1 of the modified Norwood procedure comprises an aortic arch reconstruction via a side-to-side anastomosis of the main pulmonary artery (PA) to the ascending aorta (Ao), an atrial septectomy, and a modified Blalock–Taussig shunt (arrow). Ao aorta, RV right ventricle, RA right atrium

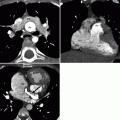
Fig. 31.2
Modified Norwood procedure, stage I. Axial (a), sagittal (b), and coronal volume-rendered (c) images. Each panel shows the neoaorta (asterisk) created by the side-to-side anastomosis of the main pulmonary artery to the ascending aorta

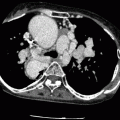
Fig. 31.3
Sano modification in the modified Norwood procedure, stage I. This approach creates a right ventricle-to-pulmonary artery conduit (arrow). The Sano shunt modification avoids the problem of competitive flow between the lungs and coronary arteries. RA right atrium, RV right ventricle

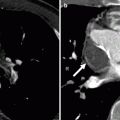
Fig. 31.4
Sano modification in the modified Norwood procedure, stage I. Panel (a), an oblique axial, and panel (b), a volume-rendered scan showing the right ventricle (RV)-to-pulmonary artery (PA) conduit (arrows). Asterisk neoaorta
Occasionally, a hybrid procedure may be performed before the Norwood operation is undertaken. This operation consists of bilateral pulmonary artery banding to limit pulmonary blood flow and stent implantation into the ductus arteriosus to maintain unrestrictive flow from the right ventricle to the systemic circulation (Figs. 31.5 and 31.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree