Chapter 37 Nuclear medicine imaging
Introduction
When radioactive substances are administered to patients, whether for diagnostic or therapeutic purposes, they are collectively referred to as radiopharmaceuticals. For diagnostic imaging these radiopharmaceuticals provide a way of visualising patterns of growth and biological activity in the organs of interest. This is achieved by imaging the distribution of radiopharmaceuticals which are selected based on their ability to be taken up in the area of interest. Abnormalities, trauma, or the effects of pathogenic invasion can be identified. The great advantage of nuclear medicine imaging is that, except in the case of trauma, physiological changes usually precede anatomical changes.1
Equipment chronology
1896 Henri Becquerel discovers radioactivity.
1930s Cyclotron invented: providing means to produce usable quantities of radionuclides. Technetium-99m (99mTc) first produced in the late 1930s.
1940s Radionuclides become available for medical use.
Early 1950s Cassen et al. produced a scintillation detector mounted in an automatic scanning gantry, which was probably the first incarnation of the rectilinear scanner.2
1953 First study involving the imaging positron emitters published.3 As cyclotrons became more available, development accelerated due to the availability of positron-emitting radionuclides that could be labelled as clinically useful molecules. Even so, positron emission tomography (PET) remained only a research tool until the late 1970s.
Late 1950s Commercial machines available: these devices allowed the acquisition of an image by tracing a collimated scintillation detector in a rectilinear pattern over the area of interest. Rectilinear scanners, however, were very slow and could not produce images of dynamic processes.
Hal Anger developed a scintillation detector, which has since become known as the gamma camera.4 This device is kept stationary and collects gamma rays over the field of view, resulting in much more rapid image acquisition than the rectilinear scanner and allowing dynamic imaging.
1960 Developed at the Brookhaven National laboratory the 99Mo/99mTc generator became commercially available. One of the earliest reported uses of 99mTc was for brain scanning.5
1963 First single photon emission tomography study published.6
This technique acquires data at a series of angular positions around the patient allowing the production of multiplanar images.
By 1964 Commercial Anger gamma camera systems available.
1967 Hounsfield develops computer algorithms for image production. These algorithms accounted for attenuation and scatter and converted the emission tomography technique to single photon emission computed tomography (SPECT). At this time reconstruction of data took several hours; however, owing to advances in computing, the same processes today take a few seconds.
1970s Radiopharmaceuticals developed allowing imaging of most organs in the body.
mid 1970s Rotating gantries developed to allow automatic SPECT acquisitions.
Late 1970s Clinical PET systems started to become commercially available.
1980s Cardiac radiopharmaceuticals became available.
Since the 1980s, systems for planar, dynamic and SPECT acquisition, have been commercially available and have been further developed and refined.
1990s Rectangular camera heads replaced circular ones to allow imaging of greater areas.
Late 1990s PET started to become routinely used as a clinical tool in the USA. A proliferation of literature started to appear to indicate that PET had a value in the diagnosis and management of certain malignant conditions, and it was not long before it was realised that PET imaging was an essential component in the management of certain cancers. The American healthcare economy then drove the PET market, and as a consequence PET scanning systems and cyclotrons became more available and at a lower cost. The increased clinical use of PET encouraged more research to be conducted into its potential applications and presently a large number of dedicated PET centres exist purely for research purposes.
Science and instrumentation
Radionuclides
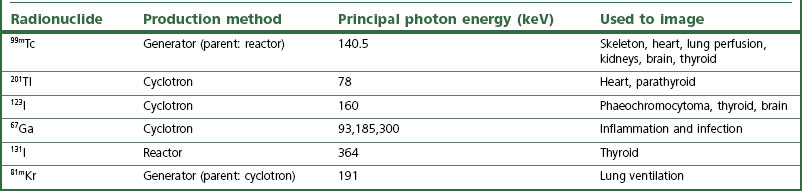
Table 37.1 gives the ideal radionuclide requirements for use in nuclear medicine imaging.
Table 37.1 Ideal radionuclide requirements for use in nuclear medicine imaging
| Property | Ideal requirements |
|---|---|
| Radiation emitted | Detection relies on radiation being emitted from the body and thus requires a penetrating form of radiation, i.e. gamma rays |
| Energy | The gamma rays must possess sufficient energy to escape the body but, conversely, their energy must be low enough to allow them to be efficiently stopped within the detector |
| Half-life | The radioactivity must be sufficient to allow good image quality throughout the duration of the imaging period. The half-life must therefore be long enough to allow this. Conversely, if the half-life is much longer than the period of imaging, this may result in a higher exposure to the patient than is necessary |
| Cost and availability | The ideal radiopharmaceutical will be cheap and readily available |
99mTc can be chemically bound to an extensive range of non-radioactive chemical compounds, which can remain chemically stable for quite long times after introduction into the patient, allowing imaging to take place. Examples of uses of 99mTc include phosphate labelled to 99mTc, which permits bone imaging, and 99mTc labelled to a chelate (e.g. diethylenediaminetetra-acetic acid) for renal imaging. Other commonly used radionuclides, and their uses in nuclear medicine imaging, are given in Table 37.2.
Radiopharmaceuticals for PET comprise radionuclides that emit positrons. The positrons lose energy in a short distance in the body and annihilate with atomic electrons to produce two 511 keV gamma-ray photons (180° apart) that allow coincidental detection of the tracer. PET radionuclides have to be produced by cyclotron. If the cyclotron is offsite, the half-life of the radionuclide must be sufficiently long to allow it to be transported to the imaging centre while enough activity remains. The most commonly used PET radionuclide is fluorine-18 (18F), which has a half-life of 1.8 hours.
Chemical component
• Using a chemical found physiologically in the organ of interest, e.g. iodine for imaging the thyroid.
• Using an analogue. This is a chemical that simulates one found physiologically, for instance thallium is a potassium analogue and thus can be used to image muscle. Similarly, fluorodeoxyglucose (FDG) is a glucose analogue and can be labelled with 18F for PET imaging to illustrate areas of high glucose metabolism, which has several clinical uses.
• Labelling cells that fight disease, thereby targeting the areas of disease, e.g. white blood cells or antibodies.
The gamma camera
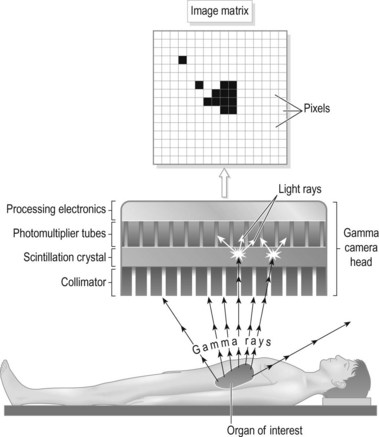
The most fundamental part of any imaging system is the detector. In the case of a gamma camera the detector is a large crystal, normally rectangular, of a scintillation material that produces a weak flash of light when radiation is absorbed, due to excitation. Flashes of light are produced when gamma rays emitted by a patient, previously administered with a radiopharmaceutical, fall on the detector (Fig. 37.1).
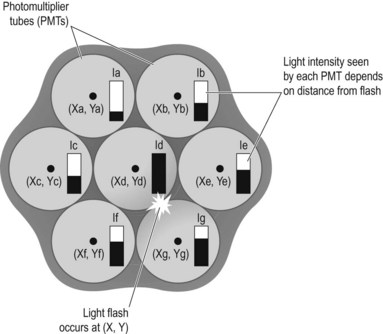
The light formed in the crystal is detected by photomultiplier tubes (PMTs) which convert the light to electronic signals whose magnitude is determined by the intensity of light reaching the PMT. There are, in fact, many PMTs packed into the space of the scintillator crystal, and those around the point of light emission will detect some amount of light, depending on their distance from that point. Those closest will detect more light and, in turn, produce a greater electronic pulse, whereas those further away will produce proportionally smaller pulses. The relative magnitude of these pulses can then be used to determine the point of light emission. The pixel count value in a corresponding location in a digital matrix can then be allocated, allowing the accumulation of an image (Fig. 37.2).
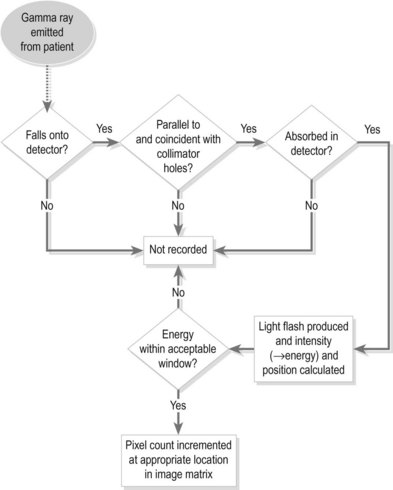
A collimator is essentially a block of attenuating material with a network of holes and is attached to the gamma camera between the detector crystal and the patient. The holes allow gamma rays travelling in a certain direction to pass through to the crystal and be detected. Gamma rays travelling in different directions are attenuated, and so the collimator effectively acts as a filter, allowing only gamma rays travelling in a known direction to contribute to the image. In this way there is a direct one-to-one mapping between the origin of the gamma ray and the position of the pixel within the image matrix. A flow diagram illustrating the process of nuclear medicine image formation is shown in Figure 37.3.
• Thickness. The thicker the crystal, the more likely the radiation will be absorbed, thereby improving efficiency. However, if the crystal is too thick there will be an increase in the drift of the light photons, adding to the uncertainty in the calculated point of interaction and hence a worsening in the spatial resolution, i.e. blurring.
• Light output. Different scintillator materials produce different amounts of light per interaction and release the light at different rates. The latter affects the duration of the processing required to assign the event to a position within the image matrix, a period known as the dead-time. Ideally a large amount of light is required in a short time. It is also essential that the light intensity produced is proportional to the energy of the radiation absorbed. This allows energy discrimination and hence the ability to disregard gamma rays outside a certain energy range, which is used to reduce scatter.
• Transparency. This factor affects the amount of light lost as it passes through the crystal before being detected by the PMTs. The more light photons that pass through the crystal and are detected by the PMTs, the more accurate the calculated point of interaction will be.
SPECT-CT systems
The CT component provides two advantages:
1 Attenuation correction. Gamma rays emitted from within the patient are attenuated by various anatomical structures before they leave the patient and are detected by the gamma camera. The amount of attenuation varies depending on the path the gamma ray travels along from its point of origin, i.e. which anatomical structures the rays have to pass through, and this will vary with the orientation of the camera during a SPECT acquisition.
2 Image fusion. The limited spatial resolution of nuclear medicine imaging, together with the efficient targeting of some radiopharmaceuticals, can result in specific uptake which is difficult to localise. On the other hand, a CT image provides good anatomical detail without the functional information. By overlaying the nuclear medicine SPECT images onto the corresponding CT image, the best of both modalities can be obtained. It is possible, for example, to see exactly which bone is affected in infection or trauma of complex areas such as the hand or foot. Hybrid systems are calibrated such that the CT and SPECT images can be accurately and consistently co-registered, and this must be checked as part of routine quality control.
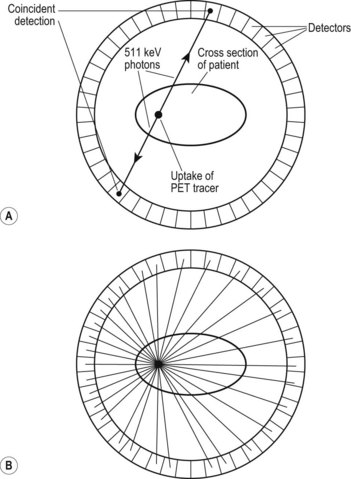
Positron emission tomography (PET)
The basic principle of PET imaging is shown in Figure 37.4A,B. PET tracers emit positrons that annihilate with electrons to form two 511 keV photons which are emitted in opposite directions. PET scanners consist of a ring of detectors in which the two photons are detected coincidentally. The point of emission in the patient must then be somewhere along a line between the two detection events. When sufficient coincident events have been accumulated, the distribution in the body is indicated by a superimposition of these lines. Reconstruction of the data produces a 3D dataset, which can be used to obtain slices through the area of interest.