CHAPTER 54 Nuclear Medicine Imaging of Myocardial Perfusion
The basic principles of MPI have been validated in animal models and clinical trials over the past several decades.1–4 Accepted clinical guidelines for use, technical aspects of quality control, and protocols for MPI stress testing and imaging for positron emission tomography (PET) and single photon emission computed tomography (SPECT) have been summarized in joint statements from the American Heart Association, American Society of Nuclear Cardiology, and American College of Cardiology,5 and the European Association of Nuclear Medicine and the European Society of Cardiology.6 This chapter highlights and summarizes the current clinical aspects of MPI. Emphasis is placed on describing the rationale and principles of MPI. The authors acknowledge variations in clinical practice based on local expertise and preference; however, space limitations do not permit a detailed discussion of all issues.
The first section reviews current clinical MPI using SPECT. Topics include radiotracers, instrumentation, procedures, and data analysis, including quantification and interpretation. Test performance and prognosis are reviewed under the general topic of interpretation. Assessment of myocardial viability with MPI also is briefly summarized (this is discussed more extensively in Chapter 55). Gated MPI to assess ventricular function is discussed in Chapter 56. Planar imaging produces lower image contrast and is reserved for special circumstances such as claustrophobic patients or patients unable to undergo SPECT imaging. An extensive review has been published.7 The second section reviews MPI with PET. The same general outline is followed in an abbreviated format with salient differences discussed between PET and SPECT.
MYOCARDIAL PERFUSION IMAGING WITH SPECT
Technical Aspects
Radiotracers
After administration, thallium 201 begins to redistribute significantly as it equilibrates with the extracellular concentration. In ischemic territories of lower MBF at rest, lower washout of radiotracer also contributes to equilibration. This more uniform myocardial radiotracer distribution at delayed (typically 4 hours) time points (“redistribution”) is deemed “reversible” compared with stress perfusion, and a functionally significant coronary stenosis can be noninvasively diagnosed. Previous studies have verified that administration of a small reinjection of thallium 201 of 37 MBq (1 mCi) at rest improves the detection of viable myocardium.8 This is a widely accepted clinical procedure to enhance the detection of viable myocardium.
Tc 99m-sestamibi is a lipophilic cationic compound. Tc 99m-tetrofosmin is a diphosphine lipophilic cationic complex of Tc 99m. Tetrofosmin is reported to have faster clearance from the liver and lungs; however, the clinical significance with respect to improved diagnostic accuracy has yet to be established. A large retrospective study has shown that Tc 99m-tetrofosmin scans are essentially equivalent to Tc 99m-sestamibi in determining prognosis in high-risk patients.9 Examples of normal Tc 99m-sestamibi studies are shown in Figures 54-1 and 54-2. A gated study showing the utility of aiding in identifying a breast attenuation artifact is shown in Figure 54-3.
Instrumentation
A modest improvement in image resolution may not be deemed necessary if the diagnostic accuracy is not significantly improved, particularly if increasing imaging time results in a higher frequency of patient motion artifacts. Advanced instrumentation and software are currently being tested, with the goal of reducing imaging time while preserving image quality. The specific details of typical clinical instrumentation, including gamma cameras, collimators, and quality assurance (QA), have been well described in a detailed review article.10
Techniques in SPECT
Indications
The American College of Cardiology Foundation and the American Society of Nuclear Cardiology jointly published guidelines for appropriateness criteria.11 The indications deemed most appropriate were those in which patients presented with intermediate or high pretest probabilities in the categories described subsequently. MPI as a screening tool in very low pretest probability patients is generally considered inappropriate.
Appropriate patient populations include the following categories: (1) detection of CAD in symptomatic or asymptomatic patients (chest pain, newly diagnosed heart failure or diastolic dysfunction, newly diagnosed arrhythmias including atrial fibrillation and ventricular fibrillation); (2) risk assessment in patients with intermediate or high pretest probability of CAD; (3) risk assessment in patients with known coronary disease; and (4) evaluation of myocardial viability. Although other indications may be warranted, the above-listed indications were judged to be most appropriate in most referred cases. Typical MPI patterns are shown in Figures 54-4 through 54-6.
A unique clinical scenario for MPI is in the evaluation of acute coronary syndromes in patients presenting to the emergency department.12 In patients without a history of prior myocardial infarction (MI) and an intermediate probability of CAD, the sensitivity for detection is very high. In this population actively having chest pain at the time of radiotracer administration, the negative predictive value of normal MPI is 99% to 100%. If the chest pain has resolved at the time of radiotracer administration, test sensitivity is modestly reduced, and current guidelines recommend repeating radiotracer administration within 2 hours of symptom abatement.12 Because Tc 99m perfusion agents do not have significant redistribution for 6 hours, imaging can be performed after resolution of chest pain and still reflects the myocardial perfusion at the time administration. In patients presenting to the emergency department with chest pain that has resolved, and in whom recent myocardial injury has been excluded by a chest pain protocol including ECG and cardiac enzymes, a subsequent stress MPI study can be safely performed to exclude functional CAD.
Pitfalls and Solutions
Quality Assurance
A QA program for gamma camera SPECT operation is essential to avoid artifacts, which could result in misinterpretation of test results. Proof of the establishment and diligent adherence to an equipment QA program is an integral part of laboratory accreditation by the American College of Radiology and the Intersocietal Commission for the Accreditation of Nuclear Medicine Laboratories. Conventional planar and specific SPECT and PET imaging QA procedures should be performed and recorded periodically. Daily QA includes ensuring correct isotope energy peak, and “daily floods” to assess gamma camera imaging field uniformity. Weekly QA includes resolution and linearity checks with “bar phantoms.” Many manufacturers include software that can automatically compute planar measurements including differential and integral flood field uniformity and intrinsic linear resolution from bar phantoms.10
Many SPECT systems include attenuation correction components, using radioactive scanning line sources, low-end CT devices, or diagnostic-quality CT. These attenuation-correcting devices require their own set of daily, weekly, and annual QA procedures. Likewise, PET and PET/CT scanners have specific QA requirements, as specified by the equipment manufacturers and as mandated by laboratory accreditation agencies. Typical PET and PET/CT QA procedures have been reviewed in a more recent publication.10
Description of Techniques and Protocols
Radionuclide Imaging Protocols
Pharmacologic Protocols—Adenosine
Adenosine is a coronary vasodilator commonly used in combination with MPI.13 This is a purine base, endogenously produced by myocardial smooth muscle and vascular endothelium. It is derived through extracellular dephosphorylation of adenosine triphosphate (ATP) and adenosine diphosphate (ADP). There are four known receptor subtypes specific for adenosine. A2A is considered a cardiac specific receptor, through which coronary vasodilation is initiated after intravenous adenosine administration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



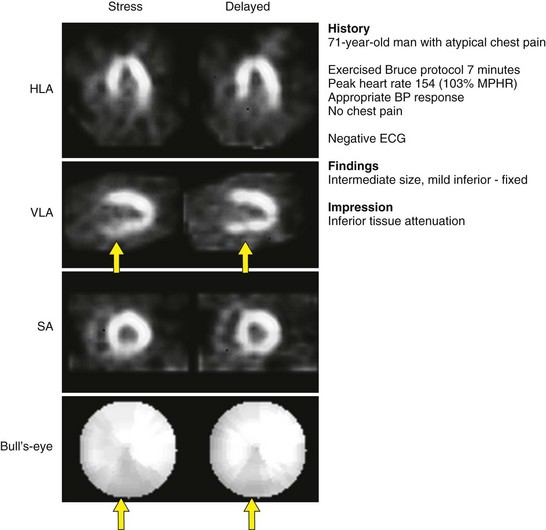
 FIGURE 54-1
FIGURE 54-1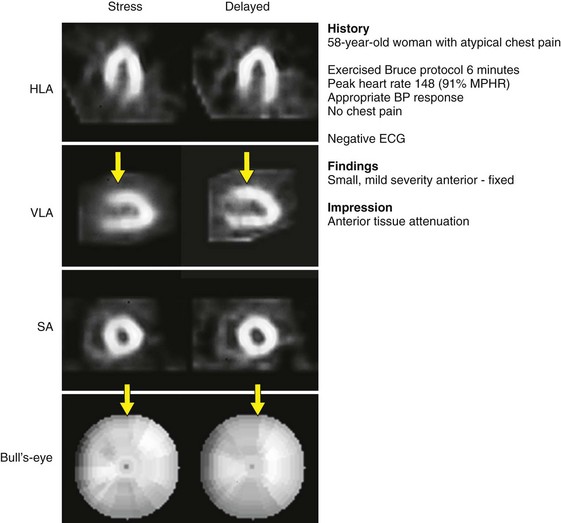
 FIGURE 54-2
FIGURE 54-2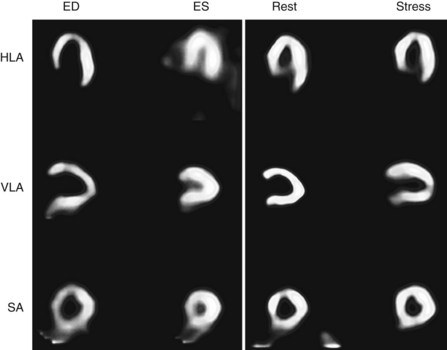
 FIGURE 54-3
FIGURE 54-3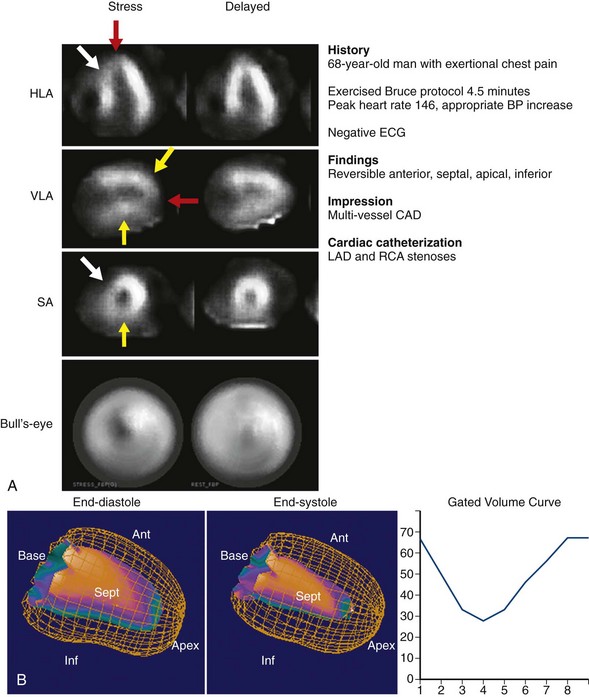
 FIGURE 54-4
FIGURE 54-4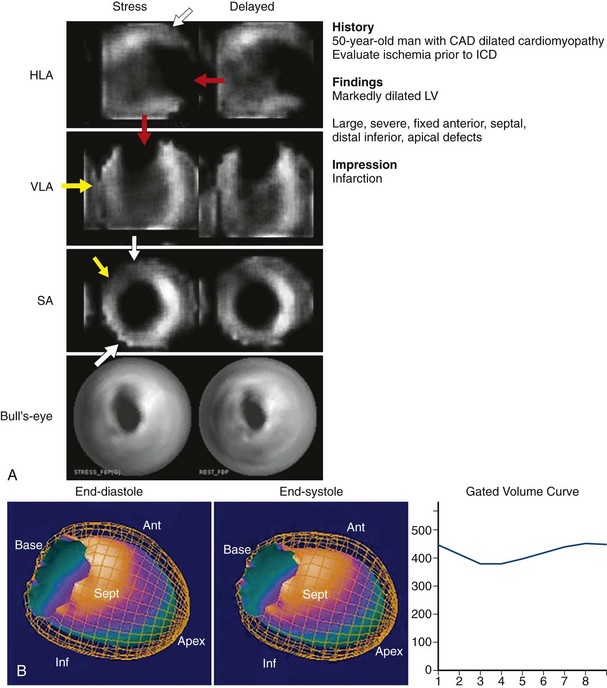
 FIGURE 54-5
FIGURE 54-5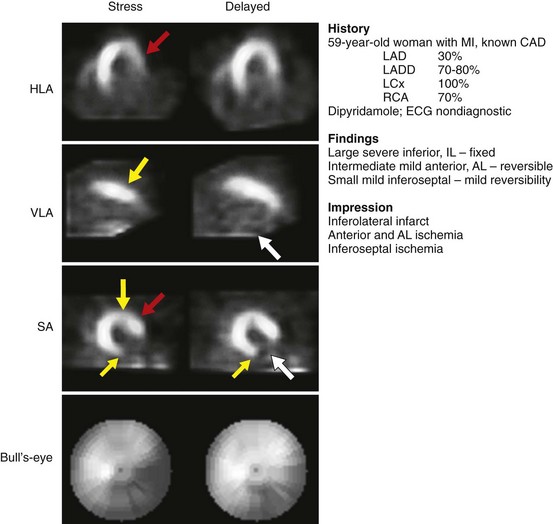
 FIGURE 54-6
FIGURE 54-6