(1)
Department of Nuclear Medicine, INHS, Asvini, Mumbai, India
Abstract
The thyroid gland is a butterfly-shaped organ situated on the anterior side of the neck. The primary function of the thyroid is the production of the hormones triiodothyronine (T3), thyroxine (T4), and calcitonin. It makes and stores essential hormones that help regulate the heart rate, blood pressure, body temperature, and rate of metabolism in the body. It also helps to control the levels of calcium and phosphorus in the blood which are needed to keep the bones strong and healthy.
4.1 Endocrine System
4.1.1 Thyroid Radioiodine Uptake
The thyroid gland is a butterfly-shaped organ situated on the anterior side of the neck. The primary function of the thyroid is the production of the hormones triiodothyronine (T3), thyroxine (T4), and calcitonin. It makes and stores essential hormones that help regulate the heart rate, blood pressure, body temperature, and rate of metabolism in the body. It also helps to control the levels of calcium and phosphorus in the blood which are needed to keep the bones strong and healthy.
The thyroid gland needs iodine to make T3 and T4. Depending upon the body requirement, production of T3 and T4 or in other words absorption of iodine is regulated by the body. Therefore uptake of iodine demonstrates the overall function of the thyroid gland. Thyroid uptake determination is the measurement of the fraction of an administered amount of radioactive iodine that accumulates in the thyroid at selected times following ingestion by thyroid uptake probe. Alternatively, thyroid uptake can be determined, though less accurately, using intravenously administered 99mTc pertechnetate and a gamma camera [1].
Indications
1.
Differential diagnosis of thyrotoxicosis based on increased and decreased % radioiodine uptake. The diseases are Graves’ disease, multinodular goiter, Hashimoto’s thyroiditis, subacute thyroiditis, thyrotoxicosis, etc.
2.
To estimate I-131 therapy dose for Graves’ disease
3.
Together with whole body thyroid cancer scans, it:
(a)
Estimate residual thyroid postsurgery
(b)
Estimate I-131 therapeutic effectiveness
(c)
Follow-up for recurrence
Instructions to the Patient
1.
The following items may be avoided as they may interfere in the concentration of radioiodine in the thyroid:
(a)
Medications such as thyroid hormones and antithyroid drugs
(b)
Iodine-containing foods (e.g., iodinated salt, fish, kelp, etc.) and medications, e.g., iodinated contrast, amiodarone, and Betadine
2.
The patient should avoid meals for at least 2 h before and 2 h after the oral dose of radioiodine.
3.
The patient should bring all previous medical documents including previous scan, reports of T3, T4, and TSH, and USG neck scan on the date of appointment.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast-feeding, they need to cease it for some time or stop it completely. For details, please contact the Radiation Safety Officer.
Procedure
1.
The measurement of thyroid uptake is usually performed 18–24 h after oral administration of the radioiodine with the help of thyroid probes. In some circumstances, it may be performed between 2 and 6 h after radioiodine ingestion (123I: 3.7–11.1 MBq or 0.1–0.3 mCi for adults and 3.7–7.4 MBq or 0.1–0.2 mCi for children below 5 years old, 131I: 0.15–0.37 MBq or 0.004–0.01 mCi, and for children below 5 years old, it is generally not used) as well.
2.
Uptakes may also be performed in conjunction with 99mTc pertechnetate. However, careful validation of this technique is required.
3.
The uptake is usually measured with 25–30 cm between the face of the crystal and the anterior neck or phantom. Neck counts, lower thigh counts (body background), counts of a calibrated standard in a neck phantom, and room background counts are preferably obtained at each counting session. Alternatively, the radioiodine dose can be counted in the neck phantom before oral administration, and the counts obtained can be corrected for decay at each patient counting session.

Fig. 4.1
Thyroid uptake measurement by a thyroid uptake probe
4.1.2 99mTc Thyroid Scan
Thyroid uptake measurement describes overall function of thyroid, whereas thyroid scan demonstrates structural function, i.e., which part of the thyroid is functioning how. Thyroid scan or scintigraphy is a procedure producing one or more planar images of the thyroid obtained within 15–30 min after intravenous injection of Tc-99m pertechnetate or 3–24 h after the oral administration of I-123 or I-131 sodium iodide [2].
Indications
1.
Determination of functional status (cold, hot) of thyroid nodule
2.
Detection of ectopic thyroid tissue (lingual thyroid)
3.
Differential diagnosis of mediastinal masses(substernal goiter)
4.
Thyroid cancer whole body scan
5.
Evaluation of congenital hypothyroidism
6.
Evaluation of a neck or substernal mass
Instructions to the Patients
1.
There is no specific instruction or preparation required for 99mTc pertechnetate scan; however, for radioiodine scans, the following items may be avoided as they may interfere in the concentration of radioiodine in the thyroid:
(a)
Medications such as thyroid hormones and antithyroid drugs
(b)
Iodine-containing foods (e.g., iodinated salt, fish, kelp, etc.) and medications, e.g., iodinated contrast, amiodarone, and Betadine
2.
The patient should avoid meals for at least 2 h before and 2 h after the oral dose of radioiodine.
3.
The patient should bring all previous medical documents including previous scan, reports of T3, T4, and TSH, and USG neck scan on the date of appointment.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, they are required to cease it for 4 h postinjection of 99m Tc pertechnetate and take advice from the Radiation Safety Officer [3]. Breast-feeding following administration of 131I should be stopped to prevent unnecessary radiation dose to the infant [1]. If 15 MBq or more of 123I is used, breast-feeding should be ceased [3].
Procedure
1.
When Tc-99m pertechnetate is used, one or more planar images of the thyroid are obtained within 15–30 min after intravenous injection of Tc-99m pertechnetate (75–370 MBq or 2–10 mCi for adults and 1.8–9.2 MBq/kg or 0.05–0.25 mCi/kg body weight of children below 5 years old).
2.
When I-123 is used, images can be obtained as early as 3–4 h after radiotracer ingestion (7.5–25 MBq or 0.2–0.6 mCi and 0.1–0.3 MBq/kg or 0.003–0.01 mCi/kg body weight of children below 5 years old). Images obtained at 16–24 h have the advantage of lower body background, but the disadvantage of a lower count rate. Interpretable images can be obtained as long as 36 h after ingestion.
3.
When I-131 is used, images should be obtained at 16–24 h after radiotracer ingestion (1.85–3.7 MBq or 0.05–0.1 mCi).
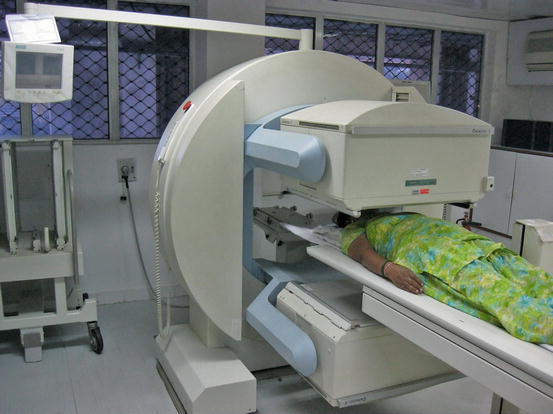
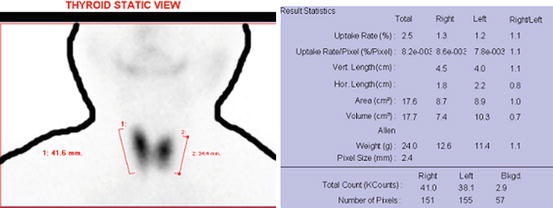
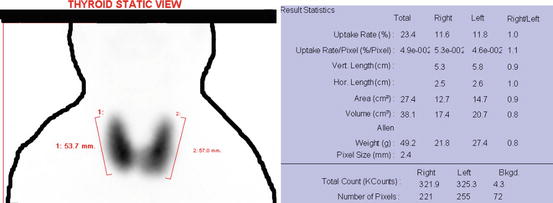


Fig. 4.2
Thyroid uptake and scan by the gamma camera

Fig. 4.3
Normal technetium thyroid scan by the gamma camera. The software provides percentage uptake of 99mtechnetium in thyroid lobe versus the injected dose of 99mtechnetium to the body. It provides vertical and horizontal length, area, volume, and weight of the thyroid too. The normal range of thyroid uptake in 99mtechnetium scan by the gamma camera is 0.3–3 %. It has been shown in the literature that normal values of 99mTc-pertechnetate uptake depend on the technique used and on the dietary intake of iodide. Each laboratory should therefore establish its own normal values [4]. In actual picture, human body outlines do not appear; it is drawn manually later on so that it can be understood by a common person

Fig. 4.4
Hyperthyroidism (Graves’ disease) in technetium scan by the gamma camera as the total uptake by the thyroid is 23.4 % shown in first row of the result statistics

Fig. 4.5
Hypothyroidism in technetium scan by the gamma camera as the total uptake by the thyroid is 0.1 % shown in the first row of the result statistics. Other black shadows seen in the image are the salivary glands at physiological and normal condition
4.1.3 MIBG (Neuroendocrine Imaging) Scan (Neuro-endocrine Imaging)
Neuroendocrinology is the study of the extensive interactions between the nervous system and the endocrine system. Neuroendocrine system governs the release of our hormones and other vital bodily elements. mIBG (metaiodobenzylguanidine) scintigraphy is used to image tumors of neuroendocrine origin, particularly those of the neuroectodermal (sympathoadrenal) system (pheochromocytomas, paragangliomas, and neuroblastomas), although other neuroendocrine tumors (e.g., carcinoids, medullary thyroid carcinoma) can also be visualized. In addition, mIBG can be employed to study the disorders of sympathetic innervations, for example, in ischemic and not ischemic cardiomyopathy as well as in the differentiation between idiopathic Parkinson’s syndrome and multisystem atrophy [5].
Indications
1.
Detection, localization, staging, and follow-up of neuroendocrine tumors and their metastases, in particular pheochromocytomas, neuroblastomas, ganglioneuroblastomas, ganglioneuromas, paragangliomas, carcinoid tumors, medullary thyroid carcinomas, Merkel cell tumors, and MEN2 syndrome.
2.
Study of tumor uptake and residence time in order to decide and plan a treatment
3.
Some other non-oncological indications
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents, including laboratory test results (plasma and urinary catecholamine dosage, CEA, 5-HIAA, NSE, chromogranin A, calcitonin, etc.) results of any other imaging studies (CT, MRI, US, X-rays) on the date of appointment.
3.
History of recent biopsy, surgery, chemotherapy, hormone therapy, and radiation therapy should be informed to the Nuclear Medicine Physician.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding and 123 I–mIBG is used, breast–feeding should be discontinued at least 48 h postinjection; and if 131 I–mIBG is used, breast-feeding should be terminated. For details, please contact the Radiation Safety Officer [5].
Preparation of Patient for the Injection
1.
Stopping of certain inferring medication
2.
Thyroid blockade by starting with stable iodine 5 days prior to injection and continued for 5 days. Thyroid blockade in adults [5]*
Compound | Daily dose |
|---|---|
Capsules | |
Potassium iodate | 170 mg |
Potassium iodide (KI) | 130 mg |
Potassium perchlorate | 400 mg |
Solution | |
Lugol 1 % | 1 drop/kg with a maximum of 40 (20 drops twice a day) |
*In children, the dose should be reduced according to standard guidelines.
Procedure
1.
The procedure involves slow IV injection of mIBG {40–80 MBq (1.2–2.2 mCi) of m131IBG or 400 MBq (10.8 mCi) of m123IBG for adults and doses may be adjusted for children} at the time of arrival on the date of appointment.
2.
Patients should be well hydrated after the radiopharmaceutical injection and void frequently.
3.
Timing of imaging: scanning with 131I-mIBG is performed 1 and 2 days after injection and can be repeated at day 3 or later. Scanning with 123I-mIBG is performed between 20 and 24 h. Selected delayed images (never later than day 2) may be useful in case of equivocal findings at day 1.The patient should be placed in the supine position.
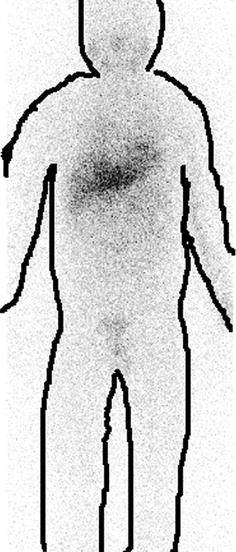
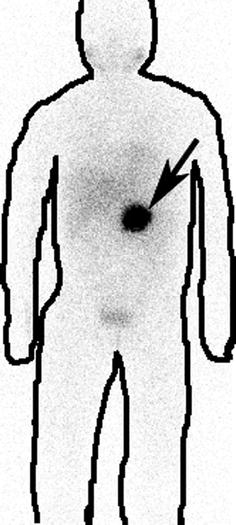

Fig. 4.6
Normal I-131 mIBG scan. Normal physiological tracer uptake is seen in the myocardium, liver, and spleen and salivary glands

Fig. 4.7
I-131 mIBG scan showing neuroendocrine tumor mass (shown by arrow)
Note: Body contours are drawn post-imaging for better understandability of nonmedical personnel
4.1.4 Medullary Thyroid Imaging by DMSA (V)
The thyroid gland is consists of cuboidal epithelial cells arranged to form small sacs known as vesicles or follicles. The vesicles are supported by connective tissue that forms a framework for the entire gland. Medullary thyroid cancer (MTC) is a form of thyroid carcinoma which originates from the parafollicular cells (C cells) which produces the hormone calcitonin. Calcitonin regulated calcium level of the body. Medullary tumors are the third most common of all thyroid cancers. It is rare and accounting for 5–10 % of all thyroid malignancies [6]. 99mTc(V) dimercaptosuccinic acid (DMSA) is used to detect MTC with sensitivity ranging from 50 % [7] to 80 % [8, 9].
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents including laboratory test results, CT, MRI, ultrasound on the date of appointment.
3.
History of recent biopsy, surgery, chemotherapy, hormone therapy, and radiation therapy should be informed to the Nuclear Medicine Physician.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy.
Procedure
1.
The procedure involves IV injection (~740 MBq or 20 mCi) of 99mTc-DMSA (V) at the time of arrival.
2.
Timing of imaging: Images are acquired 2–3 h postinjection and uptake is observed in both soft tissue and bone metastases. Static images may also be acquired if required. Single photon emission computed tomography (SPECT) imaging will increase the sensitivity of lesion detection and will also define the extent of the primary tumor more accurately [10].
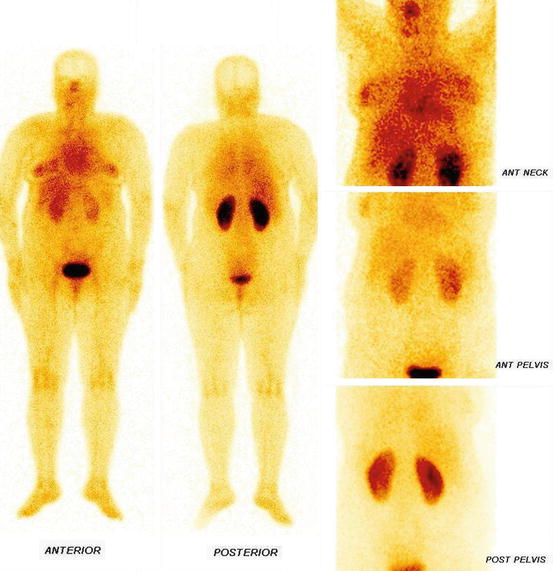

Fig. 4.8
Whole body and static images of DMSA (V)
4.1.5 T3 Suppression Test
This test is performed for diagnosing patients with borderline Graves’ disease and autonomous functioning glands [11]. Graves’ disease is an autoimmune disease where the thyroid is overactive, producing an excessive amount of thyroid hormones (serious metabolic imbalance known as hyperthyroidism and thyrotoxicosis). This is caused by thyroid autoantibodies that activate the TSH receptor, thereby stimulating thyroid hormone synthesis and secretion and thyroid growth (causing a diffusely enlarged goiter). The resulting state of hyperthyroidism can cause a dramatic constellation of neuropsychological and physical signs and symptoms [12]. Toxic autonomous nodule denotes hyperthyroidism caused by hyperfunctioning nodules in the thyroid. Functioning independently of the normal pituitary–thyroid control mechanism (thus the designation autonomous), the nodule produces excessive amounts of thyroid hormone. Unlike in Graves’ disease, the mechanism of toxic autonomous nodule is not autoimmunity.
Instructions to the Patient
1.
The following items may be avoided as they may interfere in the concentration of radioiodine in the thyroid:
(a)
Medications such as thyroid hormones and antithyroid drugs
(b)
Iodine-containing foods (e.g., iodinated salt, fish, kelp, etc.) and medications, e.g., iodinated contrast, amiodarone, and Betadine
2.
The patient should avoid meals for at least 2 h before and 2 h after the oral dose of radioiodine.
3.
Patient should bring all previous medical documents including previous scan, reports of T3, T4, and TSH, and USG neck scan on the date of appointment.
Procedure
1.
The measurement of thyroid uptake is performed 24 h after administration of the radioiodine as baseline.
2.
The patient then receives 25 mcg of T3 four times a day for 8 days.
3.
The 24 h uptake is repeated beginning on day 7.
4.
A normal response to thyroid suppression is a fall in the percentage of uptake to less than 50 % of the baseline and less than 10 % overall. An autonomous functioning gland will not show suppression.
4.1.6 TSH Stimulation Test
This test is performed to distinguish primary from secondary hypothyroidism. Primary hypothyroidism is in which the thyroid does not produce an adequate amount of T4, whereas secondary hypothyroidism develops when the pituitary gland does not release enough thyroid-stimulating hormone (TSH) that prompts the thyroid to manufacture T4. Therefore to know the function of thyroid and pituitary gland, an exogenous TSH is administered. Failure to respond to exogenous TSH is indicative of primary hypothyroidism. Patients with secondary hypothyroidism have increased radioiodine uptake after TSH stimulation [11].
Instructions to the Patient
1.
The following items may be avoided as they may interfere in the concentration of radioiodine in the thyroid:
(a)
Medications such as thyroid hormones and antithyroid drugs
(b)
Iodine-containing foods (e.g., iodinated salt, fish, kelp, etc.) and medications, e.g., iodinated contrast, amiodarone, and Betadine
2.
The patient should avoid meals for at least 2 h before and 2 h after the oral dose of radioiodine.
3.
The patient should bring all previous medical documents including previous scan, reports of T3, T4, and TSH, and USG neck scan on the date of appointment.
Procedure
1.
The measurement of thyroid uptake is performed 24 h after administration of the radioiodine as baseline.
2.
The patient then receives TSH intramuscularly.
3.
The 24 h uptake is repeated beginning on the next day.
4.
For normal patients with hypopituitarism, the uptake should double, whereas those with primary hypothyroidism show no response.
4.1.7 Perchlorate Discharge Test
This test demonstrates dissociation of the trapping and organification functions in the thyroid [11]. Organification is a process where inorganic iodide is oxidized and incorporated into tyrosyl residues (tyrosine) of thyroglobulin to form mono-iodinated tyrosine (MIT) or di-iodinated tyrosine (DIT). These MIT and DIT couple together to form triiodothyronine (T3) and thyroxin (T4). In some cases, due to some reason, iodide is taken up by thyroid but not converted to T3 or T4 (organified), i.e., it can trap iodide but cannot convert them into thyroid hormones. This study delineates the defect of the thyroid of trapping versus organification function. Organification defect can occur with congenital enzyme deficiencies, in chronic thyroiditis, and during therapy with propylthiouracil [11].
Instructions to the Patient
1.
The following items may be avoided as they may interfere in the concentration of radioiodine in the thyroid:
(a)
Medications such as thyroid hormones and antithyroid drugs
(b)
Iodine-containing foods (e.g., iodinated salt, fish, kelp, etc.) and medications, e.g., iodinated contrast, amiodarone, and Betadine
2.
The patient should avoid meals for at least 2 h before and 2 h after the oral dose of radioiodine.
3.
The patient should bring all previous medical documents including previous scan, reports of T3, T4, and TSH, and USG neck scan on the date of appointment.
Procedure
1.
The patient receives a tracer dose of radioiodine.
2.
The percentage uptake is measured at 1–2 h.
3.
One gram of potassium perchlorate is then given orally and the percent uptake is measured hourly.
4.
A washout greater than 10 % suggests an organification defect.
4.1.8 Parathyroid Imaging
The parathyroid glands are four small glands located at the four corners of the thyroid gland. They secrete parathyroid hormone which regulates the level of calcium in the blood. Primary hyperparathyroidism is characterized by increased synthesis and release of parathyroid hormone, which produces an elevated serum calcium level and a decline in serum inorganic phosphates. Asymptomatic patients are frequently identified by routine laboratory screening. The vast majority of cases of primary hyperparathyroidism (80–90 %) are due to a single hyperfunctioning adenoma. Multigland hyperplasia and double adenomas account for approximately 10 % of cases, whereas parathyroid carcinomas occur in only 1–3 % of cases of hyperparathyroidism. In general, parathyroid adenomas larger than 500 mg can be detected scintigraphically. Hyperplastic glands can be detected but with less sensitivity than adenomas [13].
Indications
1.
Parathyroid scintigraphy is specifically designed to localize parathyroid adenomas or parathyroid hyperplasia in patients with hyperparathyroidism that is determined on the basis of elevated parathyroid hormone levels in the setting of an elevated serum calcium level.
2.
Localization of hyperfunctioning parathyroid tissue (adenomas or hyperplasia) in primary hyperparathyroidism is useful before surgery to help the surgeon localize the lesion, thus shortening the time of the procedure.
3.
Localization of hyperfunctioning parathyroid tissue in patients with persistent or recurrent disease.
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all previous medical documents including previous USG on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of ~740* MBq 99mTc sestamibi or 99mTc tetrofosmin radiopharmaceutical.
2.
Early scan at 20 min and delayed image 2 h postinjection is performed under the gamma camera.
3.
This radiopharmaceutical localizes in both parathyroid tissue and thyroid tissue but usually washes out from normal and possibly abnormal thyroid tissue more rapidly than from abnormal parathyroid tissue.
4.
Three-dimensional SPECT imaging may also be performed in some cases.
Parathyroid imaging is not usually indicated in children [14].
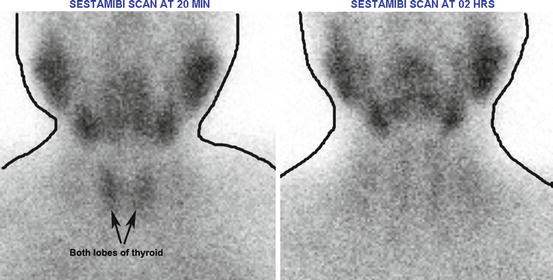
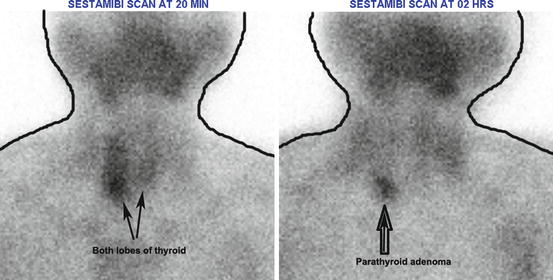

Fig. 4.9
Normal parathyroid scan (with sestamibi). Radiopharmaceutical localizes in both parathyroid and thyroid tissues but usually washes out from normal and possibly abnormal thyroid tissue more rapidly than from abnormal parathyroid tissue. Other black shadows seen in the image are normal physiological uptake of the salivary glands

Fig. 4.10
Sestamibi in parathyroid early and delayed images showing parathyroid adenoma
4.2 Skeletal System
4.2.1 MDP-Bone Scan
Bone scintigraphy is a diagnostic study used to evaluate the distribution of active bone formation in the body. Tc-99m methylene diphosphonate (MDP) has rapid blood clearance, excellent in vivo chemical stability, and a high bone-to-soft tissue ratio.
Common Indications
1.
Neoplastic disease
2.
Occult fracture
3.
Osteomyelitis
4.
Stress fracture
5.
Avascular necrosis
6.
Arthritides
7.
Sacroiliitis
8.
Reflex sympathetic dystrophy
9.
Bone infarcts
10.
Bone graft viability
11.
Otherwise unexplained bone pain
12.
Distribution of osteoblastic activity before radionuclide therapy for bone pain
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all previous medical documents including previous bone scan or X-ray on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
4.
The patient should inform the Nuclear Medicine Physician about any history of fractures, trauma, osteomyelitis, cellulitis, edema, arthritis, neoplasms, metabolic bone disease, or limitation of functions.
Procedure
1.
The patient receives an intravenous injection of the radiopharmaceutical {~740 MBq (20 mCi) for adults and 9–11 MBq/kg (250–300 μCi/kg), with a minimum of 20–40 MBq (.05–1.0 mCi) of 99mTc-MDP for pediatric patients} at the time of arrival on the date of appointment.
2.
Whole body, static, and SPECT images (if required) are obtained 02–05 h postinjection.
3.
Delayed images may be taken up to 24 h postinjection.
4.
Patients should be well hydrated after the radiopharmaceutical injection and void frequently at least up to the next 24 h.
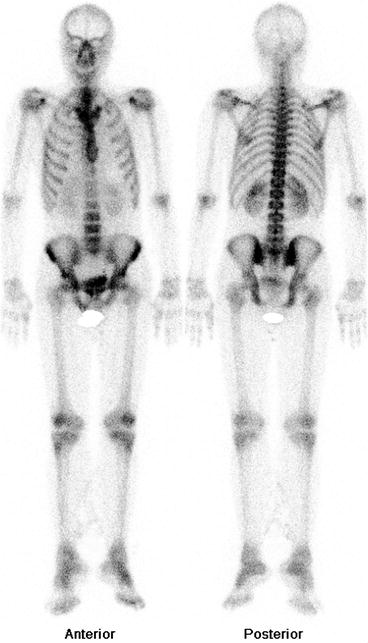
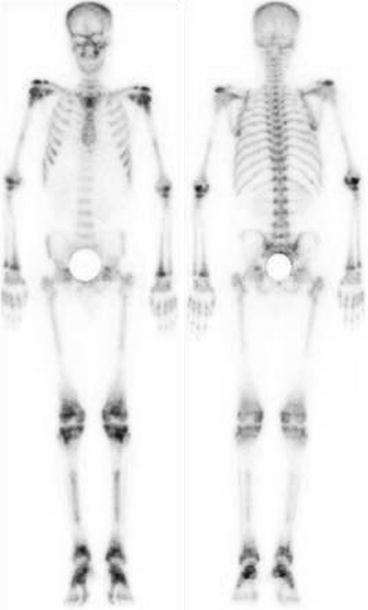
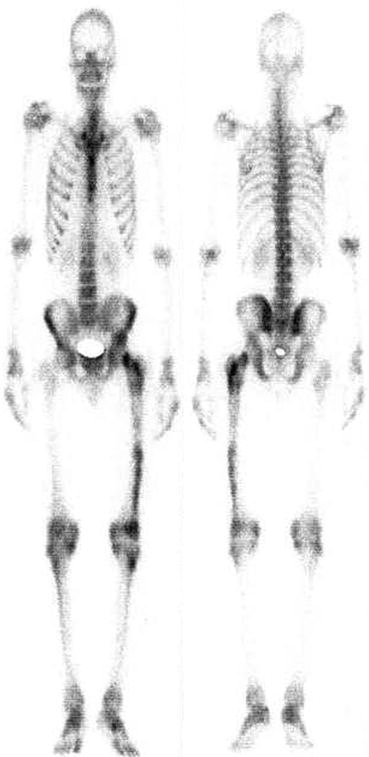
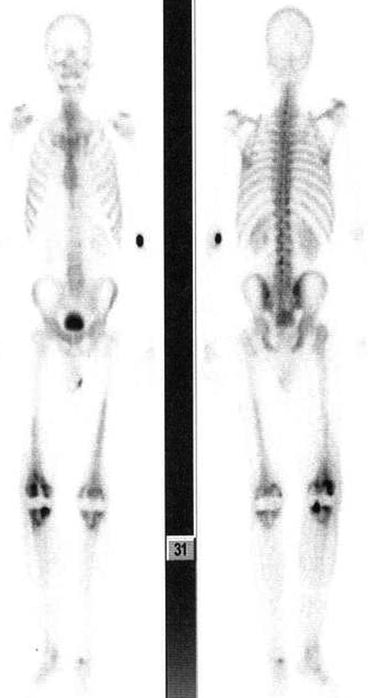
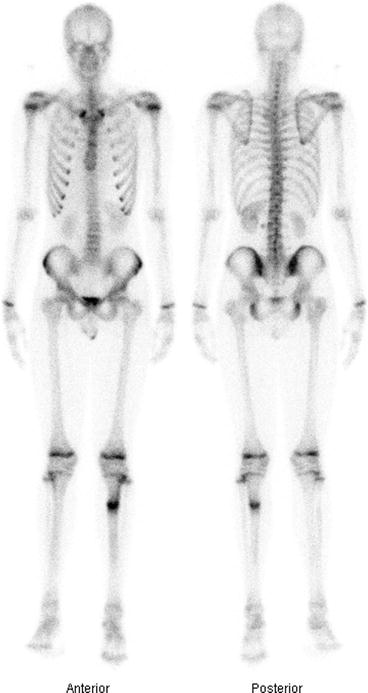
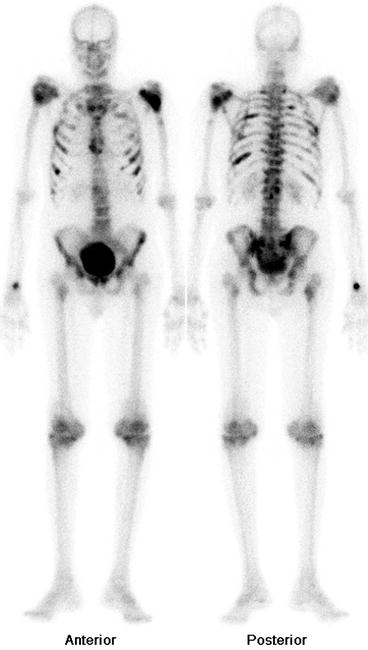
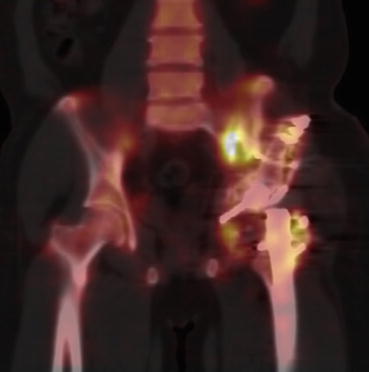
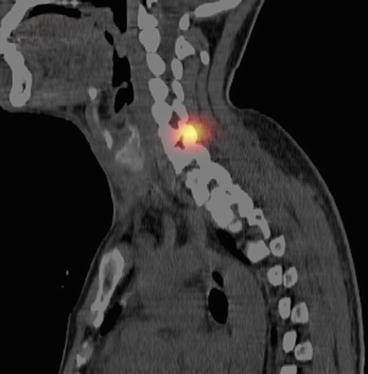
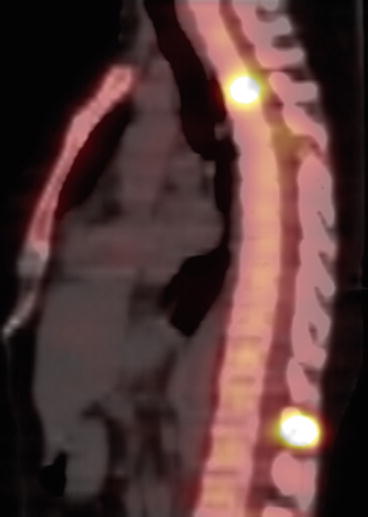

Fig. 4.11
Normal bone scan. Uniform and homogenous distribution of tracer throughout the skeleton is seen in normal bone scan. Both kidneys are also visualized

Fig. 4.12
Bone scan showing osteopetrosis in which cells involved in the decomposition and resorption of bone tissue fail to reabsorb the bone resulting in imbalance between the bone formation and breakdown

Fig. 4.13
Bone scan showing fibrous dysplasia of the femur (Lt)

Fig. 4.14
Bone scan showing joint prosthesis infection

Fig. 4.15
Stress fracture (Lt) of the tibia in an18 years old male

Fig. 4.16
Bone scan showing multiple metastases

Fig. 4.17
SPECT–CT image of hip with fracture of the (Lt) ilium and head of the femur

Fig. 4.18
SPECT–CT image of the neck with metastasis

Fig. 4.19
SPECT–CT of the spine with metastases
4.2.2 Three-Phase Bone Scan
Three-phase bone scan is helpful in the assessment of several diseases. Three phases include dynamic flow images followed by blood pool and tissue phase and finally routine whole body survey. The initial phase of Tc-99m MDP concentration in normal tissues is directly related to the blood flow and vascularity. At 3 h, Tc-99m MDP concentration in normal tissues is proportional to their calcium content, ranging from a low concentration in muscle to a high one in the bone. A similar correlation between calcium and diphosphonate retention is generally present in abnormal states.
Common Indications
1.
Osteomyelitis
2.
Stress reaction/stress fracture
3.
Evaluation of painful hip prosthesis
4.
Trauma
5.
Sacroiliitis
6.
Bone infarcts
7.
Bone graft viability
8.
Reflex sympathetic dystrophy
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all previous medical documents including previous bone scan or X-ray on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
4.
The patient should inform the Nuclear Medicine Physician about any history of fractures, trauma, osteomyelitis, cellulitis, edema, arthritis, neoplasms, metabolic bone disease, or limitation of functions.
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {~740 MBq (20 mCi) for adults and 9–11 MBq/kg (250–300 μCi/kg), with a minimum of 20–40 MBq (.05–1.0 mCi) of 99mTc-MDP for pediatric patients} under the camera. Flow and blood pool images are acquired.
2.
Whole body, static, and SPECT images (if required) are obtained 02–05 h postinjection.
3.
Delayed images may be taken up to 24 h postinjection.
4.
Patients should be well hydrated after the radiopharmaceutical injection and void frequently at least up to the next 24 h.
Three Phase Bone Scan
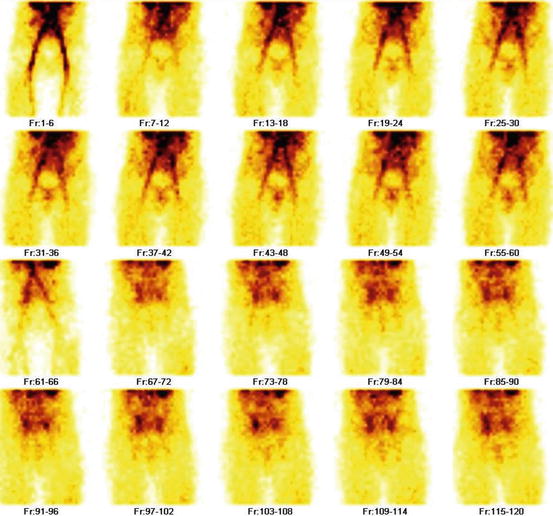
Fig. 4.20
Phase I: Blood flow images obtained in the 1st one minute of radiotracer injection

Fig. 4.21
Phase II: Blood pool image obtained at 5 min of radiotracer injection
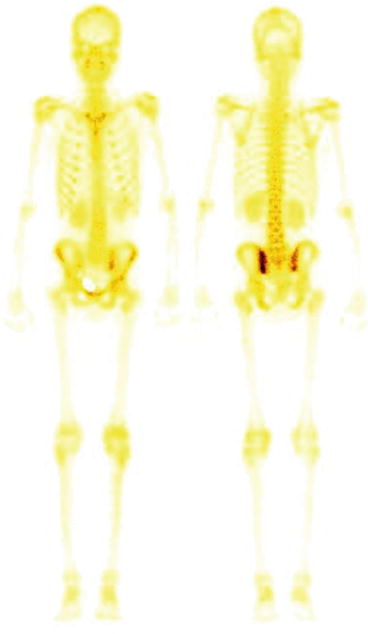
Fig. 4.22
Phase III: Whole body bone scan 3 h postinjection of radiotracer
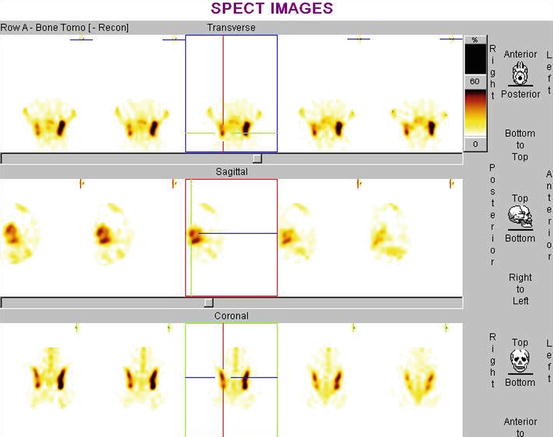
Fig. 4.23
Three-dimensional SPECT imaging. (Rt) Sacroiliac joint which is not seen in any phase
4.2.3 Bone Marrow Scan
Bone marrow scintigraphy demonstrates the extent of marrow expansion into the extremities. This scan creates a map of bone marrow to assess any changes. It is highly sensitive for bone marrow infarction and can also define the extent of involvement [15].
Common Indications
1.
Detection of bone marrow infarction
2.
Sickle cell anemia
3.
Hemoglobinopathy
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all previous medical documents including previous bone marrow scan on date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical (~740* MBq of 99mTc sulfur colloid).
2.
Whole body image and static images (if required) are obtained 3 h postinjection.
*Doses for pediatric patients may be adjusted as per standard guidelines.
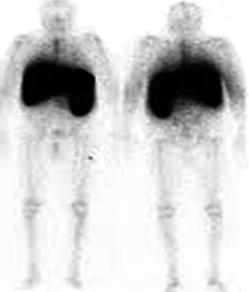
Fig. 4.24
Bone marrow scan. 5 % of the injected activity accumulates in the bone marrow, about 90 % in the liver and 5 % in the spleen. Because of the high liver uptake, it is difficult to evaluate the bone marrow in the lower thoracic and upper lumbar spine
4.3 Hepatobiliary System
4.3.1 Hepatobiliary Scan
Hepatobiliary scintigraphy is a radionuclide diagnostic imaging study (including planar imaging, SPECT, or hybrid imaging such as SPECT/CT) that evaluates hepatocellular function and the biliary system by tracing the production and flow of bile from the formative phase in the liver and its passage through the biliary system into the small intestine. Sequential (or dynamic) images of the liver, biliary tree, and gut are obtained. Computer acquisition and analysis, including pharmacologic interventions, are used according to varying indications and on individual patient’s needs [16].
Indications [16]
1.
Functional biliary pain syndromes in adults
2.
Functional biliary pain syndromes in pediatric patients
3.
Acute cholecystitis
4.
Right-upper-quadrant pain variants, as defined by the American College of Radiology Appropriateness Criteria
5.
Biliary system patency
6.
Bile leakage
7.
Neonatal hyperbilirubinemia (biliary atresia vs. neonatal hepatitis “syndrome”)
8.
Assessment of biliary enteric bypass (e.g., Kasai procedure)
9.
Assessment of liver transplant
10.
Afferent loop syndrome
11.
Assessment of choledochal cysts
12.
Calculation of gallbladder ejection fraction (GBEF)
13.
Functional assessment of the liver before partial hepatectomy
14.
Demonstration of anomalous liver lobulation
15.
Enterogastric (duodenogastric) reflux assessment
16.
Esophageal bile reflux after gastrectomy
17.
Sphincter of Oddi dysfunction
Instructions to the Patient
1.
The adult patient must have fasted for a minimum of 4 and preferably 6 h before administration of the radiopharmaceutical.
2.
No fasting for infants.
3.
Fasting for longer than 24 h (including those on total parenteral nutrition) can cause the gallbladder not to fill with radiotracer within the normally expected timeframe.
4.
The patient should bring all medical documents including previous hepatobiliary scan, bilirubin and liver enzyme, and USG of the liver and gallbladder on the date of appointment.
5.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {111–185 MBq (03–05 mCi) for adults and 1.8 MBq/kg body weight for children and neonates with a minimum of 37 MBq (01 mCi) of 99mTc mebrofenin} at the time of arrival under the gamma camera.
2.
Continuous dynamic imaging up to 60 min are performed.
3.
If required, delayed imaging up to 3–4 h may also be acquired.
4.
Delayed imaging at 18–24 h may be necessary in some cases (e.g., a severely ill patient, severe hepatocellular dysfunction, suspected common bile duct obstruction, or suspected biliary atresia).
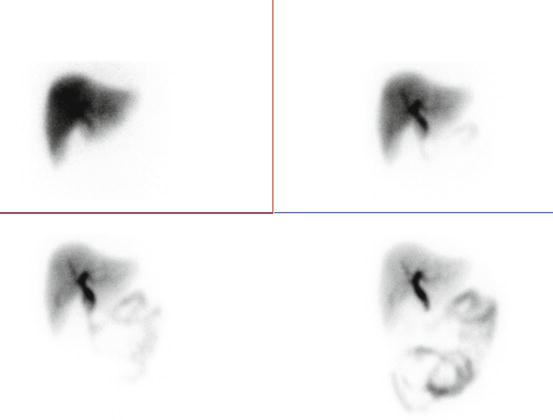
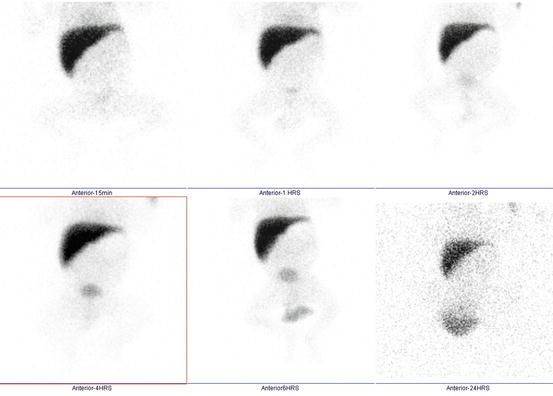

Fig. 4.25
Normal hepatobiliary scan. There is rapid visualization of the gallbladder and small intestine

Fig. 4.26
Biliary atresia where the hepatobiliary scan shows no biliary excretion into the gastrointestinal tract
4.3.2 Sphincter of Oddi Dysfunction Scan
A sphincter is a set of strong muscles that control opening and closing in the body. The sphincter of Oddi plays a part in the digestive process by controlling the flow of important chemicals such as bile from the liver and pancreatic juice from the pancreas. The sphincter of Oddi also prevents the contents of the bowel from backing up into the pancreas and bile ducts. The sphincter of Oddi dysfunction (SOD) occurs when the sphincter located in upper intestine does not open as it should. In patients suspected of sphincter of Oddi dysfunction because of persistent abdominal colic after cholecystectomy, sincalide-pretreatment cholescintigraphy can be used as a diagnostic screening test [17].
Instructions to the Patient
1.
The patient must have fasted for 2 h before the study.
2.
The patient should bring all medical documents including previous hepatobiliary scan, bilirubin and liver enzyme, and USG of the liver and gallbladder on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous infusion of sincalide 0.02 mg/kg over 3 min.
2.
15 min after sincalide infusion, the radiopharmaceutical {111–185 MBq (03–05 mCi) for adults and 1.8 MBq/kg body weight for children and neonates with a minimum of 37 MBq (01 mCi) of 99mTc mebrofenin} is injected under the gamma camera.
3.
Continuous dynamic imaging up to 60 min is performed.
4.3.3 RBC Liver Scintigraphy (for Hemangioma)
Liver hemangioma is a noncancerous (benign) mass that occurs in the liver. A liver hemangioma is made up of a tangle of blood vessels and usually asymptomatic. Liver hemangioma is sometimes called hepatic hemangioma or cavernous hemangioma. Hemangiomas are the most common benign tumor of the liver and second most common hepatic tumor, exceeded in incidence only by liver metastasis [18].
Instructions to the Patient
1.
No preparation is required for this test. There is no restriction on meal intake.
2.
The patient should bring all previous medical documents including USG of the liver on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, they are required to cease it for 12 h post-radiopharmaceutical injection and meet the Radiation Safety Officer for counseling [3].
Procedure
1.
The patient receives an intravenous injection of stannous pyrophosphate.
2.
After 10–20 min, an intravenous injection of radiopharmaceutical (~740–925* MBq (20–25 mCi) 99mTcO4 −) is given to the patient under the gamma camera.
3.
Sequential dynamic images up to 60 s are performed.
4.
Immediately a static image is acquired.
5.
If required, delayed images at 1–2 h are also acquired from multiple directions.
6.
If required, 3-D SPECT images may also be acquired.
*Doses for pediatric patients may be adjusted as per standard guidelines.
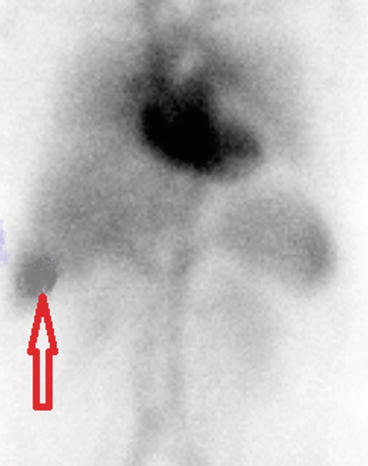
Fig. 4.27
Liver hemangioma shown by the arrow
4.3.4 Liver–Spleen Imaging
Liver and spleen scintigraphy involves the intravascular administration of radiopharmaceuticals which localize in the reticuloendothelial cells or blood pool of the liver and/or spleen when given intravenously or in the precapillary arterioles of the liver when injected through an arterial catheter into the hepatic artery. Imaging is performed with a gamma camera [19].
Indications [19]
1.
Assessing the size, shape, and position of the liver and spleen
2.
Detecting, measuring, and monitoring masses of the liver and/or spleen
3.
Differentiating hepatic hemangiomas and focal nodular hyperplasia from other liver lesions
4.
Evaluating hepatic function in acute or chronic liver disease
5.
Confirming the patency of hepatic arterial perfusion catheters and evaluating the pattern of blood flow via these catheters, including aberrant perfusion and shunting
6.
Identifying functioning splenic tissue
7.
Evaluating suspected functional asplenia
Instructions to the Patient
1.
There is no restriction on meal intake. No preparation is required.
2.
Study should not be performed immediately after barium contrast study since it may cause attenuation.
3.
The patient should bring all previous medical documents including previous liver–spleen scan, and USG of the liver.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {~111–222* MBq (03–06 mCi) 99mTc sulfur colloid}.
2.
20 min postinjection of the radiopharmaceutical, images from multiple projections are acquired.
3.
3D SPECT images may also be acquired, if required.
*Doses for pediatric patients may be adjusted as per standard guidelines.
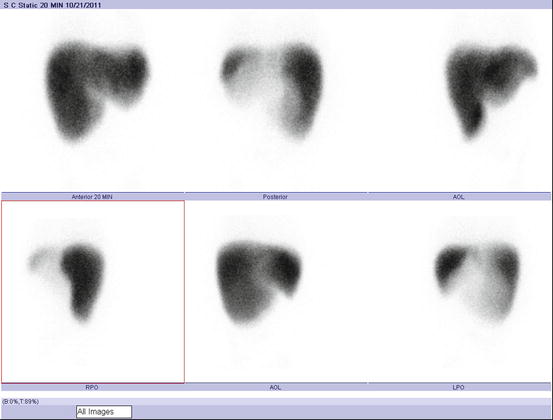
Fig. 4.28
Liver sulfur colloid image (Reprinted with permission of the American College of Radiology. No other representation of this material is authorized without expressed, written permission from the American College of Radiology. Refer to the ACR website at http://www.acr.org/Quality-Safety/Standards-Guidelines for the most current and complete version of the ACR Practice Guidelines [19])
4.3.5 Hepatic Arterial Perfusion Scintigraphy
The 99mTc macroaggregated albumin (MAA) hepatic arterial perfusion study is often performed after initial catheter placement and before subsequent courses of chemotherapy, particularly if the patient has symptoms suggestive of gastrointestinal toxicity. Effectiveness of intra-arterial chemotherapy is maximized if the entire tumor-involved liver is perfused, and side effects are minimized if there is no extrahepatic perfusion or AV shunting to the lung [20].
Instructions to the Patient
1.
There is no restriction on meal intake. No preparation is required.
2.
The patient should bring all previous medical documents on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, they need to cease it for 12 h post-radiopharmaceutical injection and contact the Radiation Safety Officer for counseling.
Procedure
1.
The patient is surgically implanted with infusion pump and catheter or percutaneously placed catheter and external infusion pump for infusion of the radiopharmaceutical.
2.
The patient receives an infusion of radiopharmaceutical {37–185* MBq (01–05 mCi) 99mTc-MAA) under the camera.
3.
Multiple images from different projections or SPECT images are acquired.
*Doses for pediatric patients may be adjusted as per standard guidelines.
4.4 Genito-urinary System
4.4.1 DTPA Renal Scan
Kidneys are responsible for regulating water and electrolyte balance, excreting waste, secreting hormones (rennin, erythropoietin), and activating vitamin D. The outer cortex contains the glomeruli and proximal convoluted tubules. 99mTc-DTPA and 99mTc-EC scans reveals glomerular filtration rate (GFR) and effective renal plasma flow (eRPF) respectively. Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Effective renal plasma flow (eRPF) is a measure used in renal physiology to calculate renal plasma flow (RPF) and hence estimate renal function.
Indications [21]
1.
Perfusion abnormalities
2.
Acute and chronic renal failure
3.
Renal transplant: rejection, obstruction, status of anastomosis
4.
Renal trauma or surgical complications
5.
Quantification of renal function: GFR/ERPF
6.
Pyelonephritis
7.
Mass vs. column of Bertin
8.
Ureteral obstruction
9.
Vesicoureteral reflux
10.
Bladder residual volume quantification
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents including previous DTPA scan, urea, creatinine, and ultrasound reports on the date of appointment.
3.
For pediatric patients below 5 years, it is advised to affix IV cannula and administer adequate sedation from the pediatric department on the date of appointment.
4.
Hydration: Hydration or volume expansion, in patients for whom there is no cardiovascular contraindication, is suggested to reduce the incidence of false-positive findings [22].
Hydration of adults: The patient should be adequately hydrated (5–10 ml/kg of body mass) commencing 0.5–01 h before the test [23].
Hydration of children: 10–15 ml/kg of N/3# or greater normal saline (with or without 5 % dextrose) for 30 min is infused before the diuretic is administered. The slow administration of fluid is continued during the remainder of the study [22].
5.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
6.
Bladder catherization may be required in some cases where it is necessary to evaluate patients with bladder pathology or in questionable cases; it is also sometimes necessary to catheterize the patient after the study to evaluate the effect of the urinary bladder. In some cases, where the kidney is not positioned and situated nearby to the bladder, catheterization may be required.
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {111–185* MBq (03–05 mCi) for adults and 3.7 MBq (0.1 mCi) per kg of body weight for children with a minimum of 37 MBq (01 mCi) of 99mTc-DTPA or 99mTc-EC} under the camera.
2.
Dynamic images are acquired for 25–30 min from the time of injection.
3.
Delayed images may be obtained, if required.
4.
Diuretics may be given at different times such as at the time of IV injection of radiopharmaceutical or at 10 min or at 20 min, depending on the case.
# N/3 normal saline means 0.9 % w/v normal saline is diluted with sterile water or dextrose to make it 0.3 % normal saline. The kidneys of pediatric patients may not be able to tolerate 0.9 % w/v of sodium load.
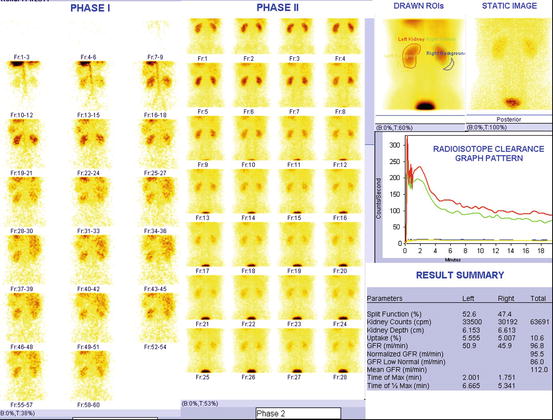
Fig. 4.29
Normal DTPA renogram. After the intravenous injection of radioisotope, images are collected over 25 min. The images are read from left to right and from top to bottom. Phase I and phase II describe flow and blood pool and excretory images. The data collected by the camera is analyzed by a computer and plotted on a time graph. Counts (how much isotope is in the kidneys) are shown on the Y-axis and time from injection is shown on the X-axis. The response of each kidney is plotted separately (color codes such as red and green are given for different kidneys). Both kidneys take up the isotope rapidly (the curves are steeply rising between 1 and 3 min). Normally at least half of the isotope is excreted and drained from the kidneys within 20 min. In result summary, different parameters such as split function of each kidney, glomerular filtration rate, time of maximum activity and clearance of half of maximum activity, etc., are produced by software
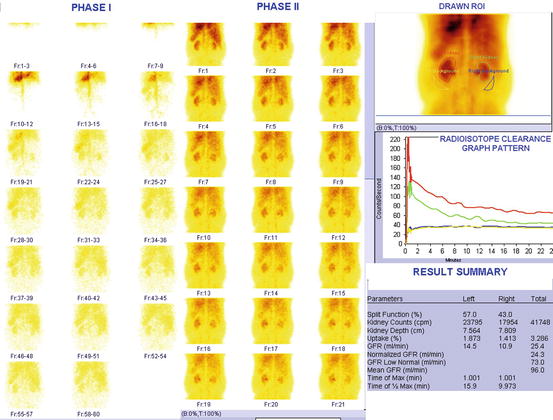
Fig. 4.30
Significantly compromised renal function. Normal values of GFR is 100 ± 20 ml/min (these values decreases with age and not applicable for children below 12 years)
4.4.2 DMSA Cortical Imaging
The renal cortex is part of the kidneys containing mostly nephrons and blood vessels. Its function is to filter the blood and remove waste products inside the body. Nephrons are the basic functional units of the kidneys, with each kidney having one million or more of these important structures. In each nephron, there is a glomerulus and a renal tubule, which is divided into sections. Renal cortical scintigraphy is used for the detection of the cortical defects of acute pyelonephritis and scarring related to chronic pyelonephritis. Cortical scintigraphy is able to detect twice as many defects as ultrasound and four times as many defects as intravenous urography [20].
Indications [20]
1.
Acute pyelonephritis
2.
Renal scarring
3.
Relative functioning renal mass
4.
Solitary or ectopic renal tissue (e.g., pelvic kidney)
5.
Horseshoe and pseudo-horseshoe kidneys
6.
Allergy to iodinated contrast agents
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents including previous DMSA scan and ultrasound reports on the date of appointment.
3.
For pediatric patients below 5 years, it is advised to affix IV cannula before intravenous injection of radiopharmaceutical and administer adequate sedation before imaging from the pediatric department on the date of appointment.
4.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {11–110* MBq (0.3–3 mCi) of 99mTc-DMSA} on the date of appointment.
2.
Three hrs postinjection of radiopharmaceutical, static images are acquired.
3.
Delayed images up to 24 h may also be obtained, if required.
*Doses for pediatric patients may be adjusted as per standard guidelines.
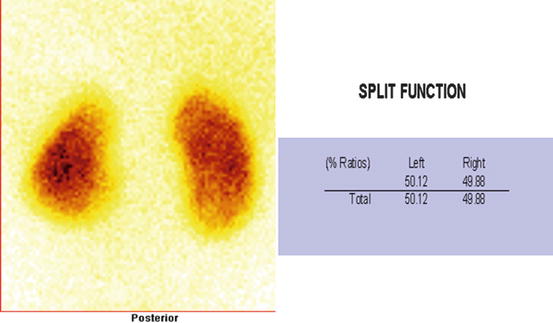
Fig. 4.31
Normal DMSA scan. Cortical scintigraphy is able to detect twice as many defects as ultrasound and 4 times as many defects as intravenous urography [24]. Findings included in this procedure are the position, size, overall morphology of functioning renal tissue, split renal function, number, and location of areas of cortical loss
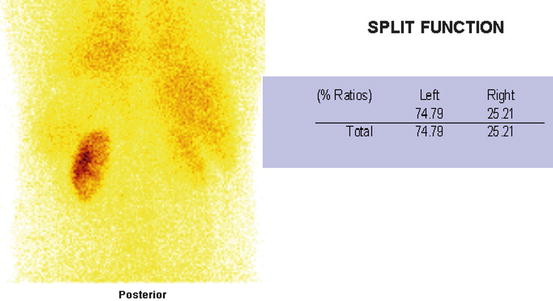
Fig. 4.32
Abnormal DMSA scan with (Rt) extensive scar with (Lt) inferior pole scar. Split function of both kidneys is ~3:1
4.4.3 Captopril Renography
Renovascular disease is a progressive condition that causes narrowing or blockage of the renal arteries or veins. These are the blood vessels that take blood to and from the kidneys. Renovascular disease includes renal artery stenosis, renovascular hypertension (RVH), and azotemic renovascular disease (ischemic nephropathy). Renal artery stenosis is the narrowing of the renal arteries and is common in non-hypertensive elderly persons associated but non-causative finding in a number of hypertensive patients. Renovascular hypertension is defined as an elevated blood pressure caused by renal hypoperfusion, usually resulting from anatomic stenosis of the renal artery and activation of the renin–angiotensin system. Azotemic renovascular disease also called ischemic nephropathy is the loss of renal function manifested by a reduction in glomerular filtration rate (GFR) or a quantitative loss of renal parenchyma as a consequence of renal artery stenosis [25].
Angiotensin-converting enzyme inhibitor (ACEI) renography is designed to be a test for RVH, not for renal artery stenosis [25]. If a patient has a moderate to high likelihood of RVH and normal renal function, ACE inhibition renography provides a logical, noninvasive, and cost-effective approach to patient management. A normal ACE inhibition renogram obviates the need for further workup; an abnormal study should lead to referral for angiography and revascularization.
Indications [25]
1.
Abrupt onset or severe hypertension
2.
Hypertension resistant to 3-drug therapy in a compliant patient
3.
Abdominal or flank bruits
4.
Unexplained azotemia in an elderly hypertensive patient
5.
Worsening renal function during antihypertensive therapy, especially with ACEIs or angiotensin II receptor blockers
6.
Grade 3 or 4 hypertensive retinopathy
7.
Occlusive disease in other vascular beds
8.
Onset of hypertension under age 30 years or over age 55 years
9.
Recurrent pulmonary edema in an elderly hypertensive patient
10.
Hypertension in infants with an umbilical artery catheter
11.
Hypertension in children
Instructions to the Patient
1.
Discontinue certain medication such as tab enalapril for 5 days, tab ramipril for 3 days, and tab losartan for 3 days before the study.
2.
The patient should bring all medical documents including previous DTPA scan, urea, creatinine, and ultrasound reports on the date of appointment.
3.
Patients should not eat a solid meal within 4 h of the study, because food in the gastrointestinal tract decreases absorption of captopril [25].
4.
A good hydration, i.e., 7 ml/kg of body weight is required, preferably 60 min before the study [26].
5.
For pediatric patients below 5 years, it is advised to affix IV cannula and administer adequate sedation from the pediatric department on the date of appointment.
6.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
Generally, 2 days protocol is followed for this investigation. On the first day, a baseline study is carried out (after stopping above medications). On the second appointment day, captopril study is performed (stopping of medications continues for this test too).
Day 1
1.
The patient receives an intravenous injection of radiopharmaceutical {111–185* MBq (03–05 mCi) for adults and 3.7 MBq (0.1 mCi) per kg of body weight for children with a minimum of 37 MBq (01 mCi) of 99mTc-DTPA} under the camera.
2.
A sequential dynamic images up to 25–30 min are acquired.
Day 2
1.
Adult patients are given 25–50 mg of tab captopril orally, whereas children are given 0.5 mg/kg (maximum 25 mg). Crushing the tablets and dissolving them in 150–250 ml of water may enhance absorption. Unless the patient has delayed gastric emptying or poor absorption from the gastrointestinal tract, 25 mg is sufficient. Blood pressure is monitored and recorded every 15 min up to 1 h. Enalapril can also be used. The recommended dose is 40 μg/kg administered intravenously over 3–5 min with a maximum administered dose of 2.5 mg. Radiopharmaceutical administration should be delayed at least 15 min after enalapril administration [25].
2.
The patient receives an intravenous injection of radiopharmaceutical {111–185* MBq (03–05 mCi) for adults and 3.7 MBq (0.1 mCi) per kg of body weight for children with a minimum of 37 MBq (01 mCi) of 99mTc-DTPA} under the camera 60 min after oral captopril or 15 min after IV administration of enalapril.
3.
A sequential dynamic images up to 25–30 min are acquired.
4.4.4 Renal Transplant Evaluation
Renal transplantation is a well-established surgical procedure. Renal scintigraphy has been used for many years in the evaluation of renal transplants and can help in the diagnosis of graft complications, leading to prompt clinical management and preventing further deterioration of renal function. Scintigraphy can assess many complications including acute rejection, acute tubular necrosis (or more properly termed, vasomotor nephropathy), vascular problems, and obstruction [27].
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents including previous DTPA scan, urea, creatinine, and ultrasound reports on the date of appointment.
3.
Adequate hydration (5–10 ml/kg of body mass commencing 0.5–01 h before the test [23]) is required.
4.
For pediatric patients below 5 years, it is advised to affix IV cannula and administer adequate sedation from the pediatric department on the date of appointment.
5.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
Procedure
1.
The patient receives an intravenous injection of radiopharmaceutical {111–185* MBq (03–05 mCi) for adults and 3.7 MBq (0.1 mCi) per kg of body weight for children with a minimum of 37 MBq (01 mCi) of 99mTc-EC} under the camera. The patient is imaged anteriorly with the camera centered over the allograft in the lower pelvis [27]. It is useful to include at least some of the native kidneys in the field of view as they may contribute to overall function. Some portion of the bladder should be seen, and the entire bladder is included on pre-void and post-void images [27].
2.
A sequential dynamic images up to 25 min are acquired.
3.
Delayed images may be obtained, if required.
4.4.5 Scrotal Scintigraphy
Scrotal scintigraphy is used to produce images of diagnostic quality that enable interpreting physician to assess the cause of acute or subacute scrotal pain. Such studies should be performed as soon as possible, so that surgery, if necessary, can be performed in a timely fashion. Scrotal scintigraphy has played an important role in the management of acute scrotal emergencies. It is primarily used to differentiate testicular torsion from other pathological conditions causing acute scrotal pain. The procedure is not indicated in evaluating cryptorchidism, tumors, or chronic inflammation [28].
Indications
1.
Differentiation of specific causes of acute and subacute scrotal pain, especially testicular torsion and epididymitis/orchitis
2.
To check viability of testis in case of injury to scrotum with acute inflammation
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all previous medical documents on the date of appointment.
Procedure
1.
Patient position: The patient should lie supine with his legs comfortably abducted. His penis should be positioned cephalad and secured in the midline to the lower abdomen. The scrotum should be suspended with lead shield placed under the scrotum or over the thighs and lower abdomen to shield extraneous counts.
2.
The patient receives an intravenous injection of radiopharmaceutical (~555–940* MBq 99mTcO4 −). The gamma camera detector is positioned anteriorly over the scrotum.
3.
Dynamic sequential images are obtained up to 10 min postinjection of radiopharmaceutical. Static images of 300–500 K counts may be acquired.
*Doses for pediatric patients may be adjusted as per standard guidelines.
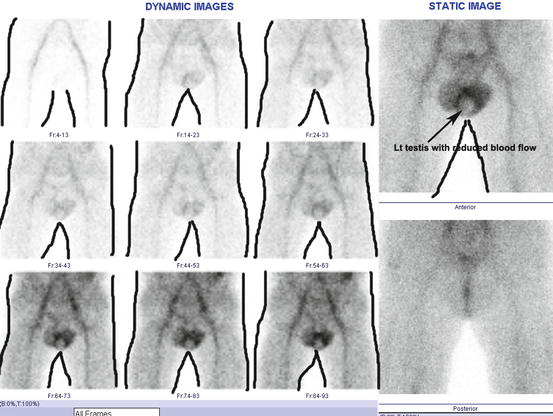
Fig. 4.33
Scrotal scintigraphy. (Lt) The testis shows reduced blood perfusion. Body contour has been drawn post-imaging for the ease of the readers, so that it can be understood well
4.4.6 DRCUG (Direct Radionuclide Cystoureterography)
Direct radionuclide cystoureterography (DRCUG) involves filling the urinary bladder with a radiopharmaceutical by direct administration via catheter or suprapubic and subsequent imaging with a gamma camera. In this technique, the radiopharmaceutical is administered aseptically into the bladder via either a urinary catheter and followed by an appropriate volume of sterile normal saline, until the bladder reaches capacity, or suprapubic injection when the bladder is full. The clinical information can also be obtained with antegrade drainage of an intravenously administered radiopharmaceutical that is excreted by the kidneys. This antegrade technique is called indirect radionuclide cystoureterography (IRCUG). IRCUG technique is described in detail in the next subchapter. Both techniques, i.e., DRCUG and IRCUG, combined is called radionuclide cystography (RNC).
Indications
1.
Diagnosis of vesicoureteral reflux in urinary tract infection
2.
Follow-up of previously diagnosed vesicoureteral reflux to assess for spontaneous resolution
3.
Evaluation of vesicoureteral reflux after medical or surgical management
4.
Serial evaluation of bladder dysfunction for reflux
5.
Diagnosis of familial reflux
6.
Quantification of post-void residual urine in the bladder
7.
Hydronephrosis
Instructions to the Patient
1.
There is no restriction on meal intake.
2.
The patient should bring all medical documents including previous USG reports on the date of appointment.
3.
Female patients should inform about their LMP, lactation, and any chance of pregnancy. If breast–feeding, there is no need to stop it for this investigation [3].
4.
Patient will be inserted with a Foley’s catheter, if he/she opt for catheter method.
Procedure
1.
The radiopharmaceutical {~18.5–37 MBq (0.5–01 mCi) of 99mTc-DTPA or 99mTc sulfur colloid for adults and 9.25–18.5 MBq (0.25–0.5 mCi) for children} is administered aseptically into the bladder via a urinary catheter and followed by an appropriate volume of sterile normal saline, until the bladder reaches capacity. Otherwise, suprapubic injection of radiopharmaceutical may be given when the bladder is full.
2.
A pre-void image is obtained.
3.
The patient is asked to void in front of the gamma camera to see any reflux.
4.
During the process of voiding, sequential dynamic images are obtained.
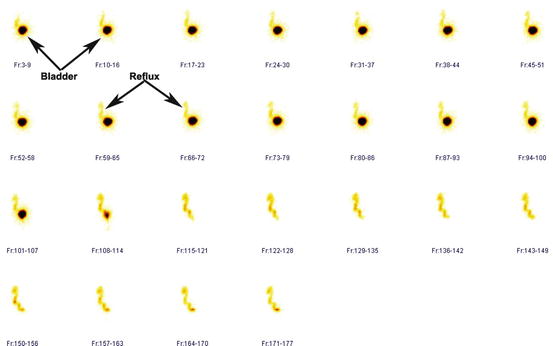

Fig. 4.34
Grade IV vesicoureteral reflux (Lt). Bladder and reflux are seen in all frames. For demonstration purpose, the bladder is marked in frames 3–9 and frames 10–16. Reflux is demonstrated in frames 59–65 and frames 66–72
4.4.7 IRCUG (In-Direct Radionuclide Cystoureterography)
Indirect radionuclide cystoureterography is usually performed as the final part of a conventional renal scan. Administered activity is the same as for renal scan with which this technique can be combined. The advantages of this technique are that it is noninvasive and it provides information about renal function. The disadvantage is a lower sensitivity than direct cystography, because the bladder may be partially filled, reflux can be detected only during the voiding phase, and it may be difficult to differentiate between reflux and residual antegrade excretion. Another disadvantage of indirect cystography is that it imparts a higher radiation dose to the patient than direct cystography. Indirect cystography should not be used if the patient is not toilet trained or has compromised renal function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




