Executive Editor: Charles W. Mitchell
© 2012 by LIPPINCOTT WILLIAMS & WILKINS, a WOLTERS KLUWER businessLWW.com
All rights reserved. This book is protected by copyright. No part of this book may be reproduced in any form by any means, including photocopying, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appearing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright.
Printed in China
Library of Congress Cataloging-in-Publication Data
p.; cm.
Includes bibliographical references and index.
Summary: “This textbook will help lay the foundation on What, How and Why to document. Legal Issues, Coding, Utilization Review and utilization management are just a few of the contents areas covered”—Provided by publisher.
ISBN 978-1-4511-0941-2 (pbk.: alk. paper)
1. Medical physics. 2. Nuclear medicine. 3. Radioisotopes. I. Title.
[DNLM: 1. Health Physics. 2. Nuclear Medicine. 3. Radioisotopes. WN 110]
R895.C47 2011
616.07’575—dc22
2011013562
Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this book and make no warranty, expressed or implied, with respect to the currency, completeness, or accuracy of the contents of the publication. Application of the information in a particular situation remains the professional responsibility of the practitioner.
The authors, editors, and publisher have exerted every effort to ensure that drug selection and dosage set forth in this text are in accordance with current recommendations and practice at the time of publication. However, in view of ongoing research, changes in government regulations, and the constant flow of information relating to drug therapy and drug reactions, the reader is urged to check the package insert for each drug for any change in indications and dosage and for added warnings and precautions. This is particularly important when the recommended agent is a new or infrequently employed drug.
Some drugs and medical devices presented in the publication have Food and Drug Administration (FDA) clearance for limited use in restricted research settings. It is the responsibility of the health care provider to ascertain the FDA status of each drug or device planned for use in their clinical practice.
To purchase additional copies of this book, call our customer service department at (800) 638-3030 or fax orders to (301) 223-2320. International customers should call (301) 223-2300.
Visit Lippincott Williams & Wilkins on the Internet: at LWW.com . Lippincott Williams & Wilkins customer service representatives are available from 8:30 am to 6 pm, EST.
10 9 8 7 6 5 4 3 2 1
To the future,
Preface
1 Basic Review
Matter, Elements, and Atoms
Simplified Structure of an Atom
Molecules
Binding Energy, Ionization, and Excitation
Forces or Fields
Electromagnetic Forces
Characteristic X-Rays and Auger Electrons
Interchangeability of Mass and Energy
2 Nuclides and Radioactive Processes
Nuclides and Their Classification
Nuclear Structure and Excited States of a Nuclide
Radionuclides and Stability of Nuclides
Radioactive Series or Chain
Radioactive Processes and Conservation Laws
Alpha Decay
Beta Decay
Gamma Decay or Isomeric Transition
Decay Schemes
3 Radioactivity: Law of Decay, Half-Life, and Statistics
Radioactivity: Definition, Units, and Dosage
Law of Decay
Calculation of the Mass of a Radioactive Sample
Specific Activity
The Exponential Law of Decay
Half-Life
Problems on Radioactive Decay
Average Life (Tav )
Biological Half-Life
Effective Half-Life
Statistics of Radioactive Decay
Poisson distribution, Standard Deviation, and Percent Standard Deviation
Propagation of Statistical Errors
Room Background
4 Production of Radionuclides
Methods of Radionuclide Production
Reactor-Produced Radionuclides
Accelerator- or Cyclotron-Produced Radionuclides
Fission-Produced Radionuclides
General Considerations in the Production of Radionuclides
Production of Short-Lived Radionuclides, Using a Generator
Principles of a Generator
Description of a Typical Generator
5 Radiopharmaceuticals
Design Considerations for a Radiopharmaceutical
Selection of a Radionuclide
Selection of a Chemical
Development of a Radiopharmaceutical
Chemical Studies
Animal Distribution and Toxicity Studies
Human or Clinical Studies
Quality Control of a Radiopharmaceutical
Radionuclidic Purity
Radiochemical Purity
Chemical Purity
Sterility
Apyrogenicity
Labeling of Radiopharmaceuticals with Technetium-99m
Technetium-99m-Labeled Radiopharmaceuticals
Technetium-99m Pertechnetate (
Technetium-99m-Labeled Sulfur Colloid
Technetium-99m-Labeled Macroaggregated Albumin (99m Tc MAA)
Technetium-99m-Labeled Polyphosphate, Pyrophosphate, and Diphosphonate
Technetium-99m-Labeled Human Serum Albumin
Technetium-99m-Labeled Red Cells
Technetium-99m-Labeled 2,3-Dimercaptosuccinic Acid (DMSA)
Technetium-99m-Labeled Diethylenetriamine Pentaacetic Acid (DTPA)
Technetium-99m-Labeled Glucoheptonate
Technetium-99m-Labeled Mertiatide (MAG3)
Technetium-99m-Labeled 2,6-Dimethyl Acetanilide Iminodiacetic Acid (HIDA) and Related Compounds (Diethyl-IDA, PIPIDA, and DISIDA)
Technetium-99m-Labeled Sestamibi (Cardiolite)
Technetium-99m-Labeled Tetrofosmin (Myoview)
Technetium-99m-Labeled Brain Imaging Agents (Exametazime [Ceretec], Hexamethylpropyleneamine Oxime [HMPAO], and Ethyl Cysteinate Dimer [ECD])
Radioiodine-Labeled Radiopharmaceuticals (131 I and 123 I)
Iodine-131- or Iodine-123-Labeled Sodium Iodide
Other Iodine-123-Labeled Radiopharmaceuticals
Compounds Labeled with Other Radionuclides
Gallium-67 Citrate
Thallous-201 Chloride
Chromium-51-Labeled Red Cells
Indium-111-Labeled DTPA
Indium-111-Labeled Platelets and Leukocytes
Indium-111-Labeled DTPA Pentetreotide (OctreoScan)
Radiolabeled Monoclonal Antibodies and Synthetic Peptides
Radioactive Gases and Aerosols
Radiopharmeceuticals for PET Imaging
18 FDG (2-deoxy-fluoro-D-glucose)
Radiopharmaceuticals in Pregnant or Lactating Women
Therapeutic Uses of Radiopharmaceuticals
Design of a Radiopharmaceutical for Therapeutic Uses
Problems and Uses
Misadministration of Radiopharmaceuticals
6 Interaction of High-Energy Radiation With Matter
Interaction of Charged Particles (10 keV to 10 MeV)
Principal Mechanism of Interaction
Differences between Lighter and Heavier Charged Particles
Range R of a Charged Particle
Factors That Affect Range, R
Bremsstrahlung Production
Stopping Power (S)
Linear Energy Transfer (LET)
Difference between LET and Stopping Power S
Annihilation of Positrons
Interaction of x- or γ -rays (10 keV to 10 MeV)
Attenuation and Transmission of X- or γ -Rays
Attenuation through Heterogeneous Medium
Mass Attenuation Coefficient, μ (mass)
Atomic Attenuation Coefficient, μ (atom)
Mechanisms of Interaction
Dependence of μ (mass) and μ (linear) on Z
Relative Importance of the Three Processes
Interaction of Neutrons
7 Radiation Dosimetry
General Comments on Radiation Dose Calculations
Definitions and Units
Radiation Dose, D
Radiation Dose Rate, dD/dt
Parameters or Data Needed
Calculation of the Radiation Dose
Step 1: Rate of Energy Emission
Step 2: Rate of Energy Absorption
General Comments on φ i (T←S)
Step 3: Dose Rate, dD/dt
Step 4: Average Dose, D
Cumulated Radioactivity
Simplification of Radiation Dose Calculations Using “S” Factor
Some Illustrative Examples
Radiation Doses in Routine Imaging Procedures
Radiation Doses in Children
Radiation Dose to a Fetus
8 Detection of High-Energy Radiation
What Do We Want to Know About Radiation?
Simple Detection
Quantity of Radiation
Energy of the Radiation
Nature of Radiation
What Makes One Radiation Detector Better Than Another?
Intrinsic Efficiency or Sensitivity
Dead Time or Resolving Time
Energy Discrimination Capability or Energy Resolution
Other Considerations
Types of Detectors
Gas-Filled Detectors
Scintillation Detectors (Counters)
Semiconductor Detectors
9 In Vitro Radiation Detection
Overall Efficiency E
Intrinsic Efficiency
Geometric Efficiency
Well-type NaI (T1) Scintillation Detectors (Well Counters)
Liquid Scintillation Detectors
Basic Components
Preparation of the Sample Detector Vial
Problems Arising in Sample Preparation
10 In Vivo Radiation Detection: Basic Problems, Probes, and Rectilinear Scanners
Basic Problems
Collimation
Scattering
Attenuation
Organ Uptake Probes
NaI(Tl) Detector
Collimator
Miniature Surgical Probes
Organ Imaging Devices
Rectilinear Scanner
11 In Vivo Radiation Detection: Scintillation Camera
Scintillation Camera
Collimators
Parallel Hole
Detector, NaI(Tl) Crystal
Position Determining Circuit (x, y Coordinates)
Display
Imaging with a Scintillation Camera
Interfacing with a Computer or All-digital Camera
Digitization in General
Digitization in the Scintillation Camera
Some Applications of Computers
Automatic Acquisition of Images
Display of Images
Analysis of the Images
12 Operational Characteristics and Quality Control of a Scintillation Camera
Quantitative Parameters for Measuring Spatial Resolution
Point-Spread Function and FWHM
Modulation Transfer Function
Resolution of an Imaging Chain
Quantitative Parameters for Measuring Sensitivity
Point Sensitivity Sp
Line Sensitivity SL
Plane Sensitivity SA
Factors Affecting Spatial Resolution and Sensitivity of an Imager
Scintillation Camera
Loss of Spatial Resolution Resulting from Septal Penetration
Variation in Spatial Resolution with Depth
Uniformity and High Count Rate Performance of a Scintillation Camera
Uniformity
High Count Rate Performance
Quality Control of Imaging Devices
Scintillation Camera
13 Detectability or Final Contrast in an Image
Parameters that Affect Detectability of a Lesion
Object Contrast
Spatial Resolution and Sensitivity of an Imaging Device
Statistical (Quantum) Noise
Projection of Volume Distribution into Areal Distribution
Compton Scattering of γ -Rays
Attenuation
Object Motion
Display Parameters
Contrast-Detail Curve
Receiver Operator Characteristic (ROC) Curve
14 Emission Computed Tomography
Principles of Transverse Tomography
Considerations in Data Acquisition
Reconstruction of the Cross Section
Attenuation Correction in Filtered Back Projection
Scatter Correction in Filtered Back Projection
Single-photon Emission Computed Tomography
Data Acquisition with a Scintillation Camera
Collimators
Other Requirements or Sources of Error
Dedicated SPECT Systems
Positron Emission Tomography
Why PET?
Principles of PET
PET Instrumentation
PET-CT and PET-SPECT
15 Biological Effects of Radiation and Risk Evaluation from Radiation Exposure
Mechanism of Biological Damage
Factors Affecting Biological Damage
Radiation Dose
Dose Rate
LET or Type of Radiation
Type of Tissue
Amount of Tissue
Rate of Cell Turnover
Biological Variation
Chemical Modifiers
Deleterious Effects in Humans
Acute Effects
Late Effects
Radiation Effects in the Fetus
Different Radiation Exposures and the Concepts of Equivalent Dose (Dose Equivalent) and Effective Dose (Effective Dose Equivalent)
Sources of Radiation Exposure
Effective Doses in Nuclear Medicine and Comparison with Other Sources of Exposure
16 Methods of Safe Handling of Radionuclides and Pertaining Rules and Regulations
Principles of Reducing Exposure from External Sources
Time
Distance
Shielding
Avoiding Internal Contamination
The Radioactive Patient
Rules and Regulations
U.S. Regulatory Agencies
Exposure or Dose Limits: Annual Limit on Intake and Derived Air Concentration
ALARA Principle
Types of Licenses
Radiation Safety Committee and Radiation Safety Officer
Personnel Monitoring
Receipt, Use, and Disposal of Radionuclides
Control and Labeling of Areas Where Radionuclides are Stored and/or Used
Contamination Survey and Radiation-Level Monitoring
Receiving and Shipping (Transport) of Radioactive Packages
Accidental Radioactive Spills
A Appendix A: Physical Characteristics of Some Radionuclides of Interest in Nuclear Medicine
B Appendix B: CGS and SI Units
C Appendix C: Exponential Table
D Appendix D: Radionuclides of Interest in Nuclear Medicine
E Appendix E: Organ Masses of a Standard Man
Answers
Suggestions for Further Reading
Index
Only gold members can continue reading.
Log In or
Register to continue
Stay updated, free articles. Join our Telegram channel
Join




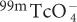 )
)