Obstetric Ultrasound–Second and Third Trimester
US is widely used in the evaluation of pregnancy with more than 70% of all pregnancies in the United States undergoing sonographic evaluation [1]. Indications for US examination are expansive and include estimation of gestational age (GA), evaluation of fetal growth, determination of fetal position, detection of multiple gestations, evaluation of fetal well-being, and detection of fetal anomalies. The American Institute of Ultrasound in Medicine provides well-accepted guidelines for the performance of obstetric ultrasound examination [2].
Guidelines for Obstetric Ultrasound Examination
Obstetric US examination in the second and third trimester should include the following standards [2], which are endorsed by the American College of Radiology [3]:
Documentation of fetal life, number, and presentation.
An estimate of the amount of amniotic fluid.
The location and appearance of the placenta and its relationship to the internal cervical os.
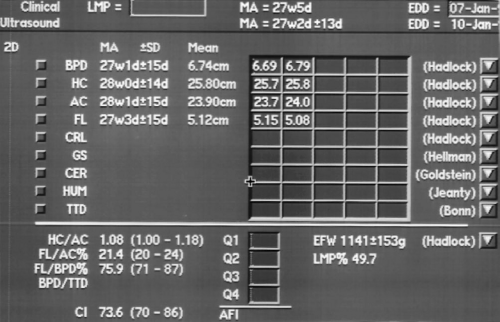
Assessment of GA using a combination of biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL).
Evaluation of the uterus and adnexa. The presence, location, and size of myomas and adnexal masses should be reported.
The study should encompass evaluation of fetal anatomy including, but not limited to, the cerebral ventricles, four-chamber view of the fetal heart, spine, stomach, urinary bladder, umbilical cord insertion site, and renal region.
Many obstetric US practices expand the evaluation to include the fetal neck, posterior fossa, extremities, ventricular outflow tracts, fetal bowel, and Doppler evaluation of the umbilical artery.
Fetal Measurements and Growth
Some of the most important aspects of obstetric care are the determination of GA and the assessment of fetal growth. By convention, clinical gestational dating is based on the first day of the last menstrual period (LMP). Conception is assumed to occur on day 14 of the menstrual cycle. A normal, full-term pregnancy is 40 weeks with a range of 37-42 weeks. Clinical dating, based on the mother’s history of LMP, is notoriously inaccurate. Sonographic dating is based on measurement of fetal parameters. Standardized charts correlate GA with measurements of fetal parameters. Serial measurements are used to document fetal growth. In the second and third trimesters, four fetal measurements are routinely used.
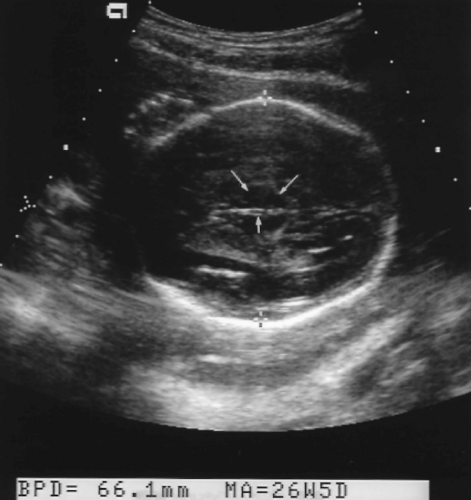
Biparietal Diameter
The BPD measurement is greatly affected by shape of the fetal head.
The BPD is determined on an axial image of the fetal head at the level of the thalamus (Fig. 7.1). The measurement is taken from the outer edge of the near cranium to the inner edge of the far cranium.
The BPD may be low for GA if the head is unusually long and narrow in shape (dolichocephaly) (Fig. 7.2A) [4].
The fetal head may be compressed by excessive transducer pressure or the molding that occurs with oligohydramnios.
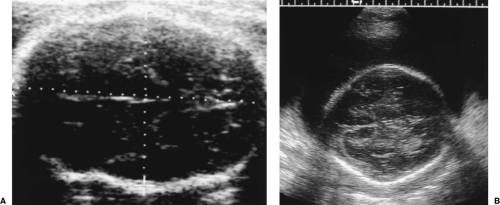
Head Circumference
The HC measurement is independent of head shape. The BPD and HC reflect growth of the fetal brain.
The HC is measured on the same axial image as the BPD. The HC is a perimeter measurement of the fetal cranium excluding subcutaneous soft tissues (Fig. 7.3).
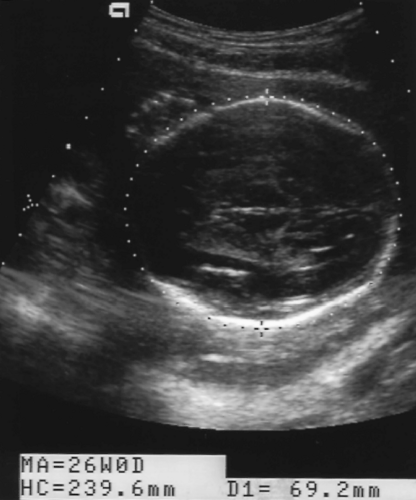
Abdominal Circumference
The AC reflects the growth of intraabdominal organs.
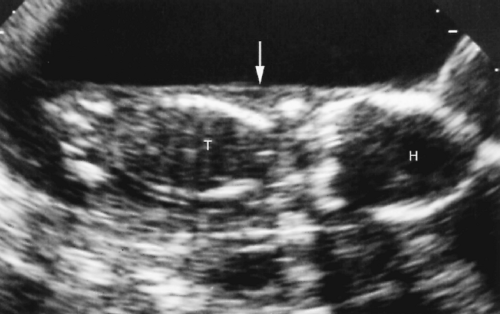
The AC is measured in axial plane at the level of the junction of the umbilical vein with the left portal vein (Fig. 7.4). The abdomen should appear round, not oval, when a true axial section is obtained. The fluid-filled stomach is routinely seen on this plane. The AC is measured as the length of the peripheral circumference of the fetal abdomen including subcutaneous soft tissues.
Femur Length
The FL serves as a monitor for growth of the long bones.
The femoral shaft is seen as a slightly curved, echogenic structure that produces an acoustic shadow. The longest dimension of the femoral shaft is measured for the FL (Fig. 7.5). The femoral epiphysis, seen as a spike on one end of the femoral shaft, is not included in the measurement. The measurement is most accurate when the femur is perpendicular to the US beam.
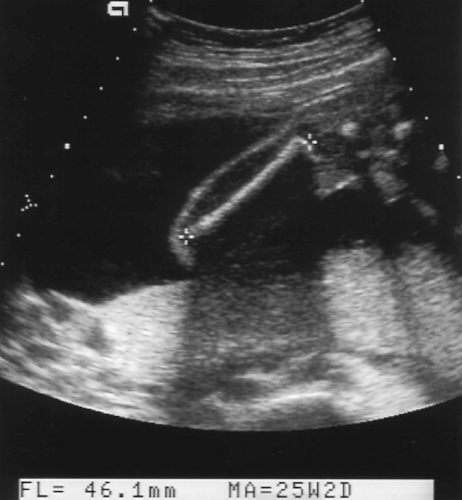 Figure 7.5 Femur Length. The femur length is the longest dimension of the shaft of the femur (between cursors, +). Note the acoustic shadow cast by the bone. |
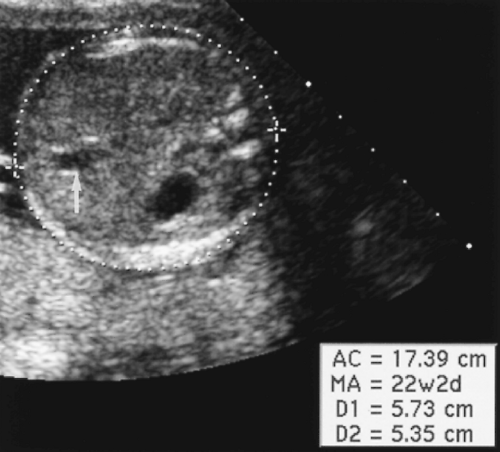
Estimated Fetal Weight
Estimated fetal weight (EFW) is used to identify fetuses that are small for GA and potentially growth retarded, and fetuses that are large for GA and may be difficult to deliver.
Assignment of GA is an interpretation based on clinical history, physical examination, and sonographic assessment. It is not just a value taken from a chart. GA is routinely determined at the time of the first US examination and is not changed thereafter. Measurements made on subsequent US examinations are compared to the GA determined on the first US examination to determine if interval growth is normal. Sonographic estimates of GA are most accurate in early pregnancy and become progressively less accurate as the pregnancy advances. Sonographic GA in the second and third trimesters is routinely based on composite age, which is the average GA determined by measurement of multiple parameters, usually BPD, HC, AC, and FL (Fig. 7.6). Fetal anomalies may make individual measurements invalid. If so, the affected measurement is excluded and composite age is determined from the remaining measurements. GA based on crown rump length in the first trimester is accurate to approximately 0.5 week. Composite GA based on the four routine measurements is accurate to 1.2 week between 12 and 18 weeks, but is accurate to only 3.1 weeks at 36-42 weeks. Measurement charts are included within the calculation packages in computer software on most US units. The range of error of each measurement is routinely listed. In the United States, most physicians use the Hadlock charts as the standards of reference, although a variety of measurement charts and formulas are available [7,8,9,10,11,12].
Intrauterine Growth Retardation
Intrauterine growth retardation (IUGR) is associated with high perinatal morbidity and mortality and an increased risk of impaired neurodevelopment. Infant mortality rate is 4-8 times greater than non-IUGR infants [13]. The diagnostic challenge is to differentiate fetuses that are pathologically small from those that are normal, but constitutionally small. Causes of IUGR are listed in Box 7.1. The approach to diagnosis of IUGR is as follows:
Box 7.1: Causes of Intrauterine Growth Retardation
| Chromosome abnormality | Maternal hypertension |
| Trisomy 13, 18, 21 | Diabetes |
| Triploidy | Smoking |
| Major congenital anomalies | Alcohol |
| Congenital infections | Cocaine |
| Rubella | Poor maternal nutrition |
| Cytomegalovirus | Maternal chronic diseases |
| Toxoplasmosis | Placental infarction |
| Teratogen exposure | Placental abruption |
| Drugs | Placental insufficiency |
Estimate the GA. Make the best estimate possible based on early US, clinical history, and physical assessment.
Compare the AC measurement to the expected AC value based on GA. An AC below the tenth percentile for GA suggests IUGR.
Compare the EFW to the expected EFW for GA. An EFW below the tenth percentile for GA suggests IUGR. If EFW is below the fifth percentile for GA, the risk of IUGR is very high.
An FL-to-AC ratio (FL/AC) >23.5 suggests IUGR.
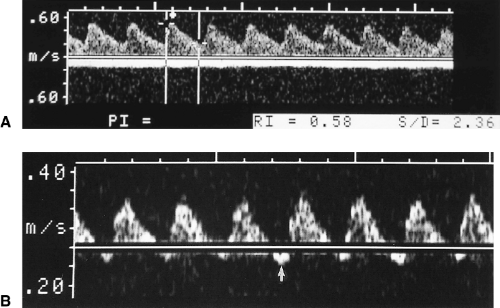
Obtain an umbilical artery spectral Doppler tracing (Fig. 7.7) [14]. A systolic-to-diastolic (S/D) velocity ratio >4 suggests IUGR [15]. Absent or reversed flow in diastolic is a highly specific sign of fetal distress, often indicative of imminent fetal death [16]. Normally the umbilical artery shows a low-resistance Doppler spectral pattern [S/D <3, resistance index (RI) <0.70]. A high resistance pattern indicates high vascular resistance within the placenta and impaired blood flow to the placenta.
Fetuses that measure small for GA but have normal Doppler studies (Fig. 7.7A) are likely to have a normal outcome [17].
Check for oligohydramnios. Low amniotic fluid volume [amniotic fluid index (AFI) <5] is found with severe IUGR [15].
The combination of IUGR with polyhydramnios is also ominous and is associated with a high incidence of chromosome abnormality (38%), major congenital anomalies, and high mortality (59%) [18].
Fetuses that are determined by these criteria to be “at risk” for IUGR are routinely followed on a weekly basis with reassessment of the listed parameters. Surveillance of fetal well-being often includes serial biophysical profiles.
Biophysical Profile
The biophysical profile is a commonly performed test used to identify fetuses that are compromised and may require expedited delivery [19]. Four “neurologic” tests are used to assess for acute hypoxia and one test (amniotic fluid) is used to check for chronic hypoxia. A score of 2 is given if the test is normal and a score of 0 is given if the test is abnormal. A total score of 8 or 10 is considered normal. Lower scores correlate with increased risk to the fetus. Abnormal results are reported only after a minimum observation period of 30 minutes.
Amniotic fluid. At least one pocket of fluid that measures 2 cm or more in a vertical plane yields a score of 2. No pockets of fluid measuring 2 cm or more in a vertical plane equals a score of 0.
Fetal movement. At least three discrete body movements of the limbs or trunk equals a score of 2. Less than three distinct body movements equals a score of 0.
Fetal tone. At least one episode of limb extension from a flexed position with return to a flexed position equals a score of 2. No extension or sluggish limb extension with failure of return to full flexion equals a score of 0.
Fetal breathing. At least one episode of breathing motion lasting at least 30 seconds equals a score of 2. No breathing motion, or breathing lasting less than 30 seconds, equals a score of 0.
Nonstress test. A normal (reactive) stress test is the observation of two or more fetal heart rate accelerations of at least 15 beats per minute (bpm) and of 30 seconds or longer duration equals a score of 2. Anything less constitutes an abnormal (nonreactive) stress test with a score of 0.
Macrosomia
Macrosomia describes babies who are large for GA. For these babies life in utero is usually uncomplicated but they are at high risk for complications during and after delivery. Many large babies are found in mothers who have gestational diabetes. Complications of macrosomia include shoulder dystocia, neurologic damage to the brachial plexus (Erb’s palsy), fractures, perinatal asphyxia, neonatal hypoglycemia, and meconium aspiration.
Macrosomia is defined as EFW above the ninetieth percentile for GA or greater than 4,000 grams.
Placenta
Normal Placenta
Normal growth and development of the fetus are critically dependent upon the normal function and integrity of the placenta. The union of chorionic villi, arising from the fertilized ovum, with maternal decidual basalis forms the normal placenta. Spiral arteries carry maternal blood to intervillous spaces between branching chorionic villi. Extensive branching provides a large surface area for exchange of metabolites [20].
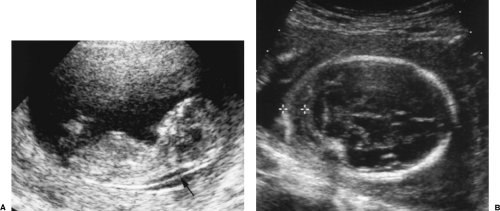
The placenta is first visualized by US at 8 weeks as a focal thickening along the periphery of the gestational sac at the site of implantation.
By 12 weeks GA, the disc shape of the placenta is evident. Its substance appears finely granular and its surface is smooth and sharply defined by the covering chorion (Fig. 7.6).
A retroplacental complex of decidual and myometrial veins creates a network of tubular sonolucent channels along the basal aspect of the placenta (Fig. 7.8). Doppler clearly defines these channels as blood vessels. Excessive transducer pressure may obliterate visualization of these normal vessels.
Normal placenta aging is manifest by the appearance of hypoechoic areas, septations, and calcifications (Fig. 7.9). Calcifications occur randomly throughout the substance of
the placenta and prominently along placental septations. Acoustic shadowing may or may not be evident. Attempts to correlate the appearance of the placenta with fetal lung maturity have been unsuccessful.
Echolucencies within the placenta commonly represent venous lakes (Fig. 7.10). Slow swirling flow may be visualized with real-time US. Flow is often too slow to demonstrate with Doppler. These venous lakes may thrombose and remain as echolucent fibrin deposits [21].
Placental thickening normally does not exceed 4 cm. Causes of an abnormally thick placenta are listed in Box 7.2 [22].
An abnormally thin placenta (<1 cm) is associated with placental insufficiency %(Box 7.3).
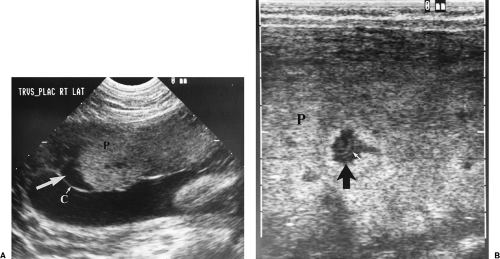
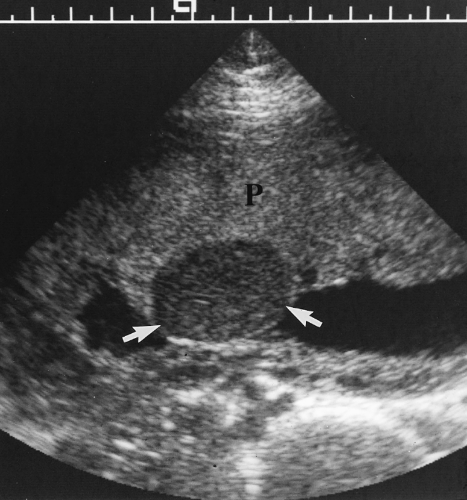
Placenta Previa
Placenta previa describes low implantation of the placenta that covers all, or a portion of, the internal os of the cervix. Placenta previa is present at term in only 0.3-0.6% of all pregnancies, but may be suggested by US in 45% of pregnancies in the first and second trimesters. Risk factors for placenta previa include previous caesarian section, previous placenta previa, multiparity, and maternal age >35 years. Complications of placenta previa are
maternal hemorrhage, premature delivery, IUGR, and perinatal death. Patients present with painless vaginal bleeding in the third trimester caused by dilatation of the cervical os, which disrupts placental blood vessels.
maternal hemorrhage, premature delivery, IUGR, and perinatal death. Patients present with painless vaginal bleeding in the third trimester caused by dilatation of the cervical os, which disrupts placental blood vessels.
Marginal previa is present when the edge of the placenta reaches or partially covers the dilating cervical os.
Complete previa is present when the os is completely covered by placenta (Fig. 7.11).
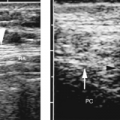
Taipale reported the risk of placenta previa at term is 5.1% when placenta previa is present on US performed at 12-16 weeks [23]. Follow-up of these patients is controversial. Some suggest that all be re-examined for placenta previa in the third trimester, whereas others recommend follow-up on only patients with risk factors or third trimester bleeding [24]. Rosati suggests following only those patients with a placenta that extends 14 mm or more beyond the internal os [25].
US diagnosis of placenta previa should always be made with the bladder empty. A full bladder distorts the appearance of the lower uterine segment and commonly creates a false appearance of previa. The cervix and placenta are easily examined with the bladder empty by a translabial approach (Fig. 7.9) [26]. Transvaginal examination is an alternative. The probe must be inserted cautiously and with direct visualization to stop at the cervix.
Vasa previa describes a membranous insertion of the cord that crosses the internal cervical os. The insertion of the cord into the placenta is velamentous. That is, the cord inserts into the peripheral membranes of the placenta rather than into the bulk of the placenta near its center. An accessory lobe of the placenta (a succenturiate lobe) may be connected to the main body of the placenta only by membranous vessels that may cross
the os. Disruption of these vessels with cervical dilatation results in rapid exsanguination of the fetus. Doppler shows blood vessels adherent to and crossing the internal os [27].
Placental Abruption
Abruption is defined as the premature separation of a normally positioned placenta from its myometrial attachment. Hemorrhage occurs from disrupted maternal vessels. Risk factors for placental abruption include maternal hypertension, toxemia, cocaine abuse, smoking, and previous placental abruption. Complications include precipitous delivery, prematurity, coagulopathy, and fetal death.
US diagnosis of abruption depends upon visualization of the resulting hematoma [28].
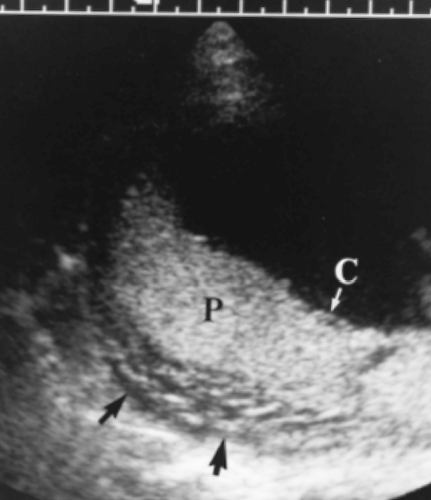
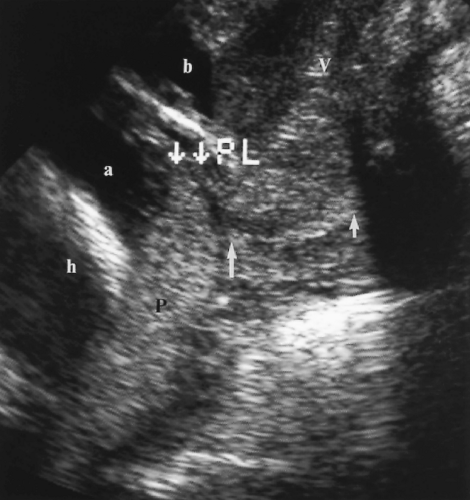
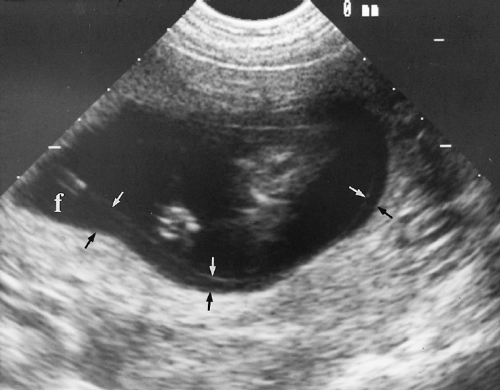
Subchorionic hemorrhage represents separation of the placenta at its margin (a “marginal” abruption). Bleeding is primarily venous and extends beneath the chorionic membrane (Fig. 7.12) (see Fig. 6.20). Large hematomas are associated with high risk of early pregnancy loss. Most subchorionic hemorrhages occur before 20 weeks gestation [29,30,31].
Retroplacental abruption is much more serious because the associated bleeding is arterial (Fig. 7.13). Extensive placental detachment disrupts placental function, causes placental infarctions, and may result in fetal hypoxia and death. Tears in the amnion allow blood to enter the gestational sac. Amniotic fluid leakage into the maternal bloodstream may cause consumption coagulopathy.
The hematoma appears as an anechoic or mixed echogenicity mass beneath the placenta and commonly extending beneath the chorion (Figs. 7.12, 7.13). The appearance of the hematoma varies with its age and physical state. The hematoma is anechoic before clot forms, isoechoic to placenta with clot formation, and becomes hypoechoic to anechoic with hemolysis 1-2 weeks after hemorrhage. When the clot is isoechoic, the placenta may appear only diffusely thickened.
Disruption of the retroplacental complex of blood vessels is an important confirmatory finding with abruption (Fig. 7.13A). Myometrial contractions and leiomyomas beneath the placenta simulate the US appearance of abruption but displace rather than disrupt the placental blood vessels.
Placenta Creta
Placenta creta describes abnormal placental invasion of the myometrium with complete or partial absence of the decidua basalis. Severity is graded as accreta with chorionic villi directly contacting the myometrium, increta with chorionic villi invading the myometrium, and percreta with chorionic villi penetrating the myometrium and invading the bladder wall. Risk factors are similar to those for placenta previa and include previous caesarian
section, increased parity, and previous uterine infection. Definitive US diagnosis may be difficult, but the diagnosis should be suggested when these findings are present [32, 33].
section, increased parity, and previous uterine infection. Definitive US diagnosis may be difficult, but the diagnosis should be suggested when these findings are present [32, 33].
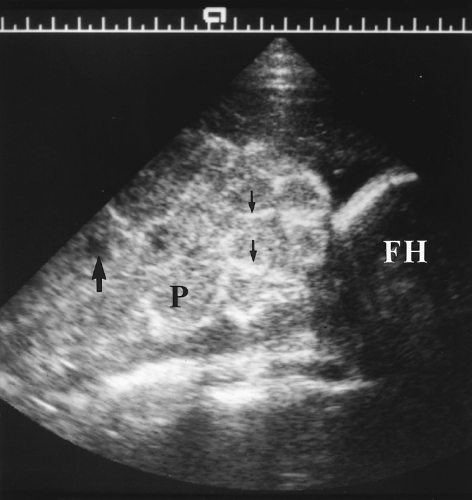
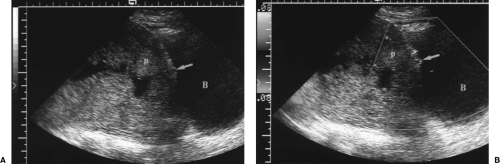 Figure 7.14 Placenta Percreta. A. Gray scale image shows a lumped-up placenta (p) with complete placenta previa. The placenta is in close proximity to the wall of the bladder (B). Note the absence of a normal retroplacental complex of blood vessels and the thin, difficult-to-visualize myometrium. The inner surface of the bladder wall has a lobulated appearance (arrow). B. Color Doppler image shows abnormal placental blood vessels (arrow) penetrating the wall of the bladder and protruding into the bladder lumen. This patient had a previous history of two cesarean sections and previous placenta previa. (See Color Figure 7.14B). |
The placenta is low lying and anterior with placenta previa often present (Fig. 7.14).
The retroplacental complex of vessels is partially or completely absent. Care must be taken to avoid compression of these vessels by excessive transducer pressure or bladder overdistention.
The myometrium underlying the placenta appears thinned (<1 mm) or absent.
The bright reflection of the serosa separating the uterus from the bladder is absent.
Color Doppler may show contiguous blood vessels extending from the myometrium into the bladder wall (Fig. 7.14B). The abnormal blood vessels may cause focal elevations of the bladder mucosa.
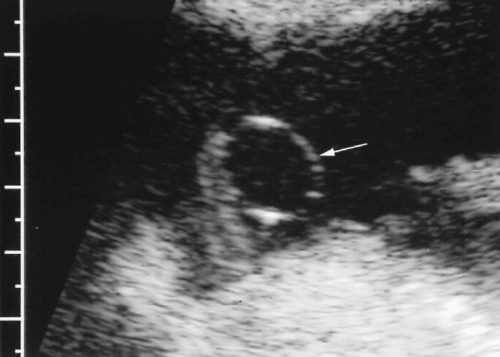
Placental Chorioangioma
Chorioangioma is a benign tumor of the placenta sometimes classified as a hamartoma. They are found in 1% of placentas pathologically but most are small and not clinically significant [21]. US detects only the larger lesions which are associated with elevation of maternal serum alpha-fetoprotein (MS-AFP).
Chorioangiomas appear as well-defined, hypoechoic, or mixed echogenicity masses within the placenta, often near the cord insertion site (Fig. 7.15) [34]. Detected chorioangiomas are usually 1-5 cm in size.
Spectral Doppler is diagnostic with demonstration of vessels within the tumor with blood flow pulsating at fetal heart rate.
Placental hematomas may have a similar appearance but have no blood flow on Doppler US.
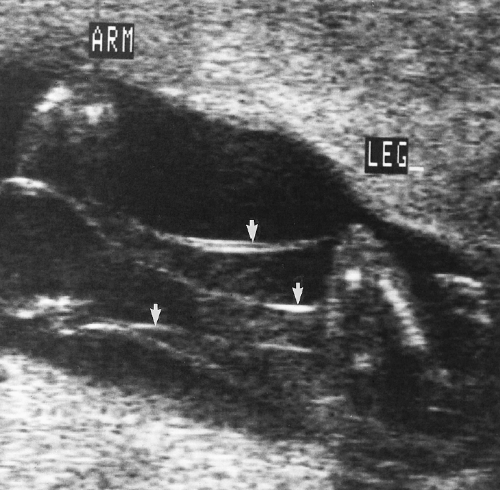
Umbilical Cord
Normal Umbilical Cord
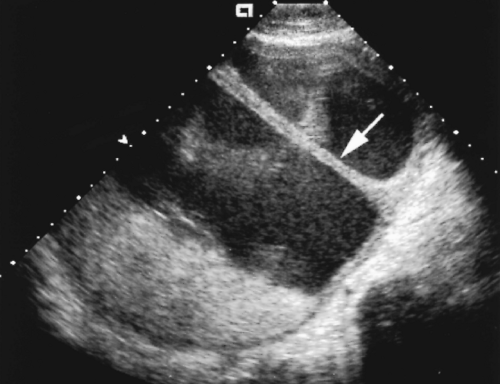
The normal umbilical cord contains two arteries and a single vein (Fig. 7.16A, C). The cord is easily visualized in amniotic fluid. Color Doppler shows its spiraling configuration.
Confirmation of one or two umbilical arteries is easily made by examining the fetus and demonstrating the umbilical arteries coursing on both sides of the bladder (Fig. 7.16C, D) [35].
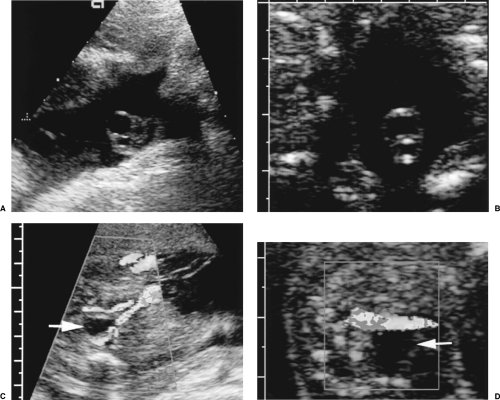 Figure 7.16 Umbilical Cord. A. A normal three-vessel umbilical cord has two smaller arteries carrying blood from the fetus to the placenta and one larger vein carrying oxygenated blood and nutrients from the placenta to the fetus. B. A two-vessel umbilical cord has a single artery and a single vein. Color flow images of the bladder (arrow) confirms the presence of two, C, or one, D, umbilical arteries coursing adjacent to the bladder from the fetal hypogastric arteries to the umbilicus. Imaging the bladder is useful when optimal cross sectional images of the cord cannot be obtained. (See Color Figures 7.16C, D). |
Two-Vessel Umbilical Cord
A single umbilical artery is found in up to 1% of pregnancies. A two-vessel cord is associated with chromosome anomalies and a variety of fetal malformations [36].
The cord contains one artery and one vein. Only a single umbilical artery is seen adjacent to the bladder in the fetus (Fig. 7.16B, D).
A careful and complete anatomic survey of the fetus is indicated to detect developmental anomalies. Most centers do not perform amniocentesis if no anomalies are found [37].
Nuchal Cord
The cord encircles the fetal neck in up to 25% of pregnancies. Multiple encircling loops and a tight nuchal cord put the fetus at risk for fetal distress especially during labor [35].
Color Doppler provides a high sensitivity for detecting nuchal cord.
Masses in the Umbilical Cord
Umbilical cord cysts arise from remnants of the omphalomesenteric duct (vitelline duct) or allantoic duct (Fig. 7.17). Fetal anomalies are commonly associated. Cysts are usually small (4-6 mm).
Tumors of the umbilical cord are exceedingly rare and include hemangiomas and teratomas [35].
Hematomas of the cord usually occur only with manipulation or puncture of the cord (percutaneous umbilical cord sampling).
Uterus and Cervix
Leiomyomas in Pregnancy
Leiomyomas are the most common pregnancy-associated pelvic tumor present in up to 3% of pregnant women. Myomas are associated with bleeding, premature uterine contractions, malpresentation, and obstruction during labor. Approximately 15% of myomas will increase in size during pregnancy. The remainder remain stable in size or disappear because of progressive stretching of the myometrium [38, 39].
Leiomyomas appear as spherical masses that distort the contour of the myometrium, and have heterogeneous, usually decreased, echogenicity compared to myometrium. Calcifications may be present. Color Doppler shows myometrial vessels displaced around the myoma [40].
Uterine contractions must be differentiated from myomas. Contractions are transient, although they may persist for 1 hour. Contractions are homogeneous and isoechoic to myometrium. They bulge the inner, but usually not the outer, uterine wall. Color Doppler shows no vessel displacement in the area of a contraction [40].
Myomas with a volume >200 cm3 show a higher rate of complications than smaller myomas [39].
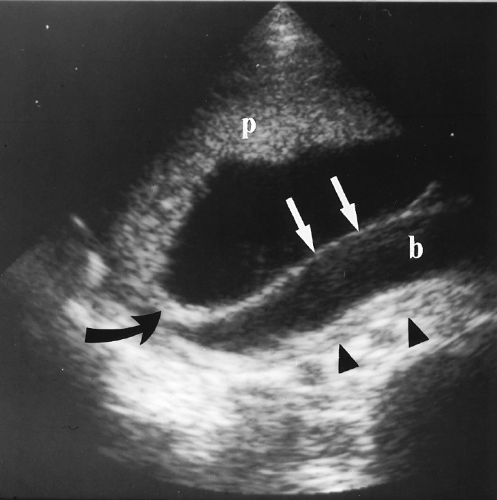
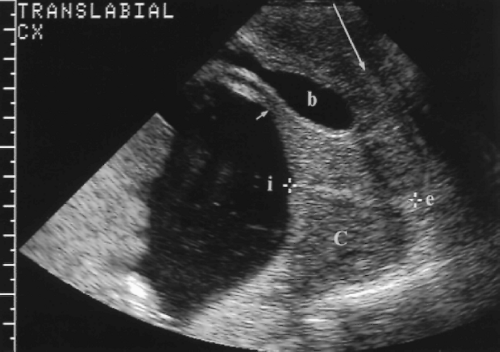
Normal Cervix
The normal cervix remains closed during pregnancy to physically retain the fetus in utero and to prevent ascending infection of the uterus [24].
A translabial approach is optimal to evaluate the cervix with the bladder empty (Fig. 7.18) [41]. A 3.5-4.0-MHz sector transducer is routinely utilized. The transducer is covered by a sterile glove or condom and is placed directly on the patient’s labia. The US beam is directed down the long axis of the vagina and is perpendicular to the cervix.
The normal cervix is seen as a hypoechoic cylinder contiguous with the myometrium (Fig. 7.18). The cervical canal is seen as an echogenic line commonly surrounded by a hypoechoic zone. The internal os is defined by the point at which the amniotic sac meets the cervical canal. The external os is interpreted as the point at which echogenic cervical canal is no longer visible [41].
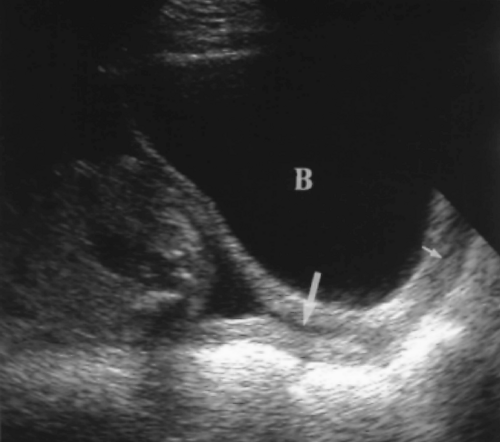
The length of the cervix is measured along the endocervical canal from internal to external os. The normal cervix has a mean length of 3.4-3.7 cm and a minimum length of 2.5 cm [42]. A full bladder compresses the lower uterine segment and falsely elongates the cervix (Fig. 7.19). Cervical length is most accurately measured with the bladder empty (Fig. 7.18).
Incompetent Cervix
A shortened cervix is predictive of cervical incompetence with its associated high risk of premature delivery. Cervical incompetence is responsible for approximately 16% of premature deliveries [24].
The cervix is considered abnormally short when the closed endocervical canal is <2.5 cm in length (Fig. 7.20A) [42]. Cervical length >3.0 cm effectively excludes pre-term delivery.
Fluid within the cervical canal indicates dilatation of the cervix. Measurement of the distance between the anterior and posterior wall of the cervix indicates the degree of dilatation. The closed portion of the endocervical canal, measured between the dilated portion of the cervix and the external os, is considered the functional cervical length.
Membranes may bulge into or through the cervical canal. When membranes bulge into the vagina, delivery is inevitable (Fig. 7.20B).
Amniotic Fluid
Normal Amniotic Fluid
Amniotic fluid protects the fetus from injury, allows growth and fetal movement, and is essential for normal lung maturation. In early pregnancy, fluid in the amnion and chorionic spaces is a filtrate of the membranes. After 16 weeks GA, nearly all of the amniotic fluid originates from fetal urination. Fetuses with bilaterally impaired renal function have profound oligohydramnios by 18 weeks. Amniotic fluid is removed from the amniotic cavity primarily by fetal swallowing.
The volume of amniotic fluid rises steadily to a maximum at 22 weeks GA and stays at that level until delivery [43]. Accurate measurement of amniotic fluid volume by US is
difficult and most sonographers rely on estimating fluid volume subjectively based on their experience [44].
Normal fluid volume allows free but not unlimited movement of fetal limbs. Fluid is seen around the fetus but both the anterior and posterior uterine walls are in contact with the fetus.
The amniotic fluid index (AFI) is widely utilized in an attempt to be more objective. The index is determined by measuring the vertical height of the deepest fluid pocket in each quadrant of the uterus and summing the 4 measurements. Umbilical cord and fetal parts are excluded from any measurement. The normal range of the AFI is 5-20 cm.
Fine particulate matter suspended in the amniotic fluid is usually a normal finding. It usually represents vernix in the third trimester, but may also result from blood or meconium [45].
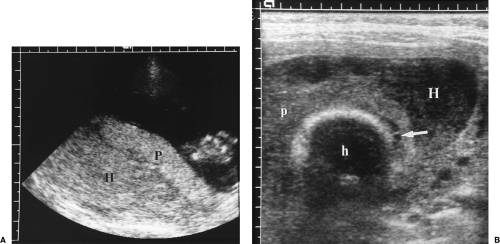
Oligohydramnios
Oligohydramnios indicates abnormally low volume of amniotic fluid. Oligohydramnios is associated with increased perinatal morbidity. Causes include reduced urine output (renal agenesis, bilateral renal dysplasia, and urinary tract obstruction), IUGR, premature rupture of membranes, and post-term pregnancy.
AFI below 5 cm is indicative of oligohydramnios (Fig. 7.21A).
Polyhydramnios
Polyhydramnios is an excessive volume of amniotic fluid. Polyhydramnios is associated with maternal diabetes under poor control, gastrointestinal and central nervous system anomalies, lethal skeletal dysplasias, and chromosome anomalies. Severe polyhydramnios may cause abdominal pain, breathing difficulty, premature rupture of membranes and premature
delivery. Polyhydramnios is commonly idiopathic and many fetuses with mild polyhydramnios will have a normal outcome.
delivery. Polyhydramnios is commonly idiopathic and many fetuses with mild polyhydramnios will have a normal outcome.
AFI above 20 cm is indicative of polyhydramnios.
A single fluid pocket >8 cm in vertical height indicates polyhydramnios.
The fetus is observed to float in the excessive fluid (Fig. 7.21B).
Fluid is seen anteriorly between the fetus and the anterior uterine wall.
Membranes
Chorioamniotic Separation
The amnion is seen separately from the chorion until 16 weeks GA when the two membranes normally fuse (see Chapter 6). Persistent separation of chorion and amnion is a normal variant but may also result from amniocentesis. No morbidity is associated with persistent separation [46].
The amnion appears as a thin, undulating membrane suspended in fluid (Fig. 7.22). While the chorion is tightly adherent to the surface of the placenta, the amnion is commonly seen separately over the placenta.
Amniotic Sheets
Amniotic sheets develop over uterine synechiae that cross the uterine cavity. Synechiae result from previous uterine surgery or infection. Membranes drape over the synechiae as the pregnancy develops and the sac enlarges [47, 48].
Visualized membranes are thick because they consist of two layers of chorion and two layers of amnion. The membrane forms a shelf-like structure about which the fetus moves freely (Fig. 7.23).
Visualization of a free edge is diagnostic. The edge is usually rounded and is thicker than the membrane because it includes the synechiae.
A Y-shaped splitting of the membrane is seen at the attachment to the uterine wall as the double layers of chorion and amnion separate [47, 48].
No fetal anomalies are associated with amniotic sheets. The risk of malpresentation or poor pregnancy outcome is not increased [49].
Amniotic Band Syndrome
Amniotic band syndrome is a common cause of fetal malformations present in 1 in 1200 births [50]. Disruption of the amnion allows the fetus to enter the chorionic cavity where it becomes entangled by sticky fibrous septa. Entrapment of random fetal parts results in amputations and slash defects that are nonembryological in distribution.
Amniotic bands appear as septa of varying thickness that may produce a spider web appearance (Fig. 7.24). Extension of bands to entangled limbs may be visualized. Absence of US visualization of amniotic bands does not exclude the diagnosis of amniotic band syndrome.
Extremities are most frequently involved with asymmetric amputations that involve only one digit or the entire limb. Focal constriction may result in marked lymphedema of the peripheral limb.
Head defects include asymmetric anencephaly, encephaloceles away from the midline, and facial clefts that extend beyond normal boundaries.
Truncal deformities may include the chest and abdomen and resemble gastroschisis with associated angulation deformities of the spine. The distal spine may be amputated.
Chromosome Anomalies and Biochemical Screening
Approximately 4 infants per 1000 live births have a significant chromosome abnormality [51]. The incidence of chromosome abnormalities is much higher in spontaneous abortions and stillbirths. Aneuploidy is the state of having an abnormal number of chromosomes (more or less than 46). The risk of aneuploidy increases with increasing age. Triploidy refers to the presence of a complete extra set of chromosomes (69 chromosomes). Most triploidies result in spontaneous abortions. Triploidy is associated with partial hydatidiform mole. Trisomy is the state of having three, instead of the usual pair, of any one chromosome. Trisomy implies aneuploidy with 47 chromosomes. Common trisomies are trisomy 21 (Down’s syndrome), trisomy 18 [52], and trisomy 13 [53].
Initial screening for chromosome abnormalities was based on maternal age alone [54]. The risk of Down’s syndrome is 1 in 910 at age 30 and progressively increases to 1 in 110 at age 40. At age 37 the risk is 1 in 240, which is approximately equal to the risk of fetal loss associated with amniocentesis, approximately 1 in 200. If amniocentesis is offered to all pregnant women age 35 and older, approximately 40-60% of Down’s syndrome fetuses will be detected [54]. Additional efforts at detection of chromosome anomalies are based on biochemical screening of maternal serum and on US examination.
Maternal Serum Alpha-Fetoprotein
Biochemical screening for fetal abnormalities initially focused on alpha-fetoprotein (AFP) as a marker of neural tube and other fetal anatomic defects [55]. AFP is a glycoprotein made initially in the yolk sac and later by the fetal liver. MS-AFP levels vary with GA and peak at 28-32 weeks. Fetal tissue not covered by skin leaks AFP into the amniotic fluid where it is absorbed into the maternal bloodstream. Levels of AFP in maternal blood that exceed 2.5 multiples of the median (MOM) for GA are considered abnormal for singleton pregnancies. Levels >4.5 MOM are abnormal for multiple fetus pregnancies. MS-AFP screening detects 98% of open spina bifida defects and anencephaly. See Box 7.4 for a summary of abnormalities associated with elevated MS-AFP.
Low levels of MS-AFP (0.63-1.0 MOM) are associated with increased risk of Down’s syndrome. However the sensitivity of low MS-AFP for Down’s is only approximately 21%. This has led to the use of “triple marker” maternal serum screening.
Box 7.4: Causes of Elevated Maternal Serum Alpha-Fetoprotein
| Neural tube defect | Placental abnormalities |
| Open spina bifida (98% of cases) | Chorioangioma |
| Anencephaly (98%) | Placental hematoma |
| Cephalocele | Placental insufficiency |
| Abdominal wall defects | Chromosome abnormalities |
| Gastroschisis (77–90%) | Trisomy 13 |
| Omphalocele (40%) | Trisomy 18 |
| Amniotic band syndrome | Turner’s syndrome (XO) |
| Triploidy | |
| Incorrect menstrual dating | |
| Multiple fetus gestation | |
| Fetal demise |
Box 7.5: US Markers of Down’s Syndrome
“Soft” Markers
Short femur length– Also seen with skeletal dysplasias [135].
Echogenic bowel (≥ bone)–Also seen with bowel obstruction, intrauterine infection, cystic fibrosis, and bowel ischemia. Finding implies a risk of adverse outcome of the pregnancy [114].
Choroid plexust cyst– Has a higher association with trisomy 18. The presence of a choroid plexus cyst is an indication for detailed fetal anatomic survey for additional anomalies (Fig. 7.47) [54, 56].
Nuchal fold– Nuchal translucency ≥3 mm in the first trimester, or nuchal skin thickness ≥6 mm in the second trimester is highly associated with the presence of Down’s syndrome [60, 61]. Increased nuchal fold thickness in the second trimester is also associated with Turner’s syndrome (XO).
Echogenic focus in cardiac ventricle– Associated risk of Down’s syndrome is increased by as much as fourfold [101, 136, 137].
Mild renal pyelectasis– (≥4 mm at 15-20 weeks) Is seen in 17-25% of fetuses with Down’s syndrome [138].
“Hard” Markers
Duodenal atresia– Is the most common intraabdominal abnormality associated with Down’s syndrome. However, US can rarely make the diagnosis before 22 weeks GA.
Congenital heart disease– Up to 50% of fetuses with Down’s syndrome have congenital heart disease, most commonly complete atrioventricular canal or ventricular septal defect.
Triple Marker Screening
Expanded AFP, or triple marker, screening measures the levels of AFP, human chorionic gonadotropin, and unconjugated estriol in maternal serum. Race, ethnicity, maternal age, and accurate determination of GA are used to determine the risk level for chromosome abnormality.
Sonographic Screening for Down’s Syndrome
US findings associated with Down’s syndrome are summarized in Box 7.5. The presence of an abnormal nuchal fold, a “hard” marker, or two soft markers is considered an indication for parental counseling and consideration of amniocentesis for chromosome analysis [56, 57]. Use of sonographic markers in a high-risk population can identify up to 75% of fetuses with Down’s syndrome [58]. A number of centers recommend against counseling if only one “soft” marker is present because of the high price of parental anxiety for what is most likely to be an insignificant problem [54, 59].
Nuchal thickening is one of the strongest predictive signs of chromosome abnormality. Nuchal thickness is measured on the transcerebellar view. Nuchal thickness >3 mm in the first trimester [60] or >6 mm in the second trimester is associated with the presence of chromosome anomalies (Fig. 7.25) [61].
Multiple Pregnancy
Mortality of twins is 15% higher than mortality of singleton pregnancies. The incidence of congenital anomalies is increased 4-fold in twin pregnancies. In addition, twins may experience a variety of disorders that are unique to twinning.
Approximately 1 in 80-90 deliveries involves twins. Triplets are much less common, approximately 1 in 6400 births. Quadruplets occur in 1 in 512,000 births.
Placentation
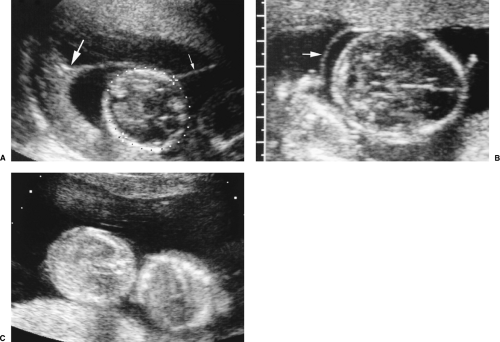
The risks of twin pregnancies are related to the type of twinning [62]. Dizygotic twins are always dichorionic, diamniotic. The placentation of monozygotic twins depends upon the timing of the division of the fertilized ovum that results in twinning. When division is early (first 3-4 days), the twins are dichorionic, diamniotic (1/3). When division occurs at 4-7 days, the twins are monochorionic, diamniotic (2/3). When division occurs after 8 days, the twins are monoamniotic (<1%) and at highest risk for adverse outcome. Division later than 13 days results in conjoined twins.
In the first trimester, the presence of a thick membrane separating the two sacs indicates dichorionic twins. If the membrane is thin or not seen, the pregnancy is monochorionic. Because the amnion membrane separating the twins may not be evident, amnionicity cannot be reliably determined until the second trimester. The presence of two yolk sacs indicates diamniotic twins. If only one yolk sac is present, the pregnancy is monoamniotic [63].
In the second trimester the pregnancy is dichorionic if the twins are of different gender or if two separate placentas are clearly visualized.
A single placental mass may consist of two abutting placentas or a single placenta that supplies both twins. A thick membrane, consisting of two chorions and two amnions, separating the twins indicates dichorionic twinning. A thin membrane, consisting of two layers of amnion, separating the twins indicates monochorionic, diamniotic twins. An easy-to-see membrane of 1-2 mm is considered “thick.” A difficult-to-see membrane is “thin.” Differentiation of thin and thick is obviously subjective and not very accurate (Fig. 7.26).
The “twin-peak sign” is more definitive [64]. A beak-like tongue of placenta protrudes between the two double-membranes of dichorionic diamniotic twins (Fig. 7.26A). The single chorion of a monochorionic pregnancy prevents this protrusion of placenta between membranes.
Visualization of an amnion in the second trimester is definitive for diamniotic pregnancy. However, when the amnion is not visualized it still may be present. Diagnosis of monoamniotic twinning requires additional findings such as entangled umbilical cords or fetal parts (Fig. 7.26C).
Growth of Twins
Twins closely parallel the growth of singletons in the first and second trimesters, so the same growth charts can be used [65, 66]. In the third trimester, the weight gain of normal twins slows and charts specific to twins may be used for more accurate assessment. IUGR affects approximately 25% of twin pregnancies.
GA is determined at the first US examination by averaging the composite GA of the twins, provided they are not grossly discordant. Subsequent US examinations are compared to the first examination for growth. Once established, the GA of the twins is not changed [62]. If the first US examination is performed late in pregnancy, FL measurements appear to be most reliable for determining GA.
A 15% difference in EFW or AC between the twins is considered evidence of significant growth discordance.
Twin-Twin Transfusion Syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree