Introduction
Ocular point-of-care ultrasound (POCUS) can rapidly identify ocular emergencies, including the diagnoses of lens detachment/dislocations, retinal detachment, vitreous detachment, vitreous hemorrhage, ocular infections, the presence of foreign bodies, and secondary signs of elevated intracranial pressure. In a fast-paced clinical practice, a fully dilated ophthalmologic examination may be impractical, and studies have shown that many practitioners lack the clinical skills to perform it effectively. The benefits of performing an ocular ultrasound include ease of use, low cost, sensitivity for identifying pathology, lack of ionizing radiation, and ability to perform imaging at the bedside. POCUS offers few risks, with the exception in the setting of a possible globe rupture, and should not cause significant discomfort to the patient when done correctly. Therefore ultrasound can supplement the acute eye examination, and can accurately detect a range of important eye disorders and attempt to rule out emergent conditions.
Anatomy
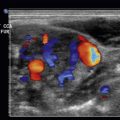
To fully utilize the benefits of the ocular ultrasound, it is imperative to have an accurate understanding of the anatomy of the eye ( Fig. 5.1 ). The white, outermost layer of the eye is the sclera, which surrounds the entire globe and serves as a protective layer. The limbus creates the junction between the sclera and the cornea, which is the transparent part of the eye that covers the colored iris. The pupil allows light to enter the eye. The anterior chamber (AC) is the space between the lens and the cornea. The AC contains aqueous humor produced by the ciliary body, which is continuous with the colored iris, and the choroid, which is the vascular bed for the retina. With ultrasound, this will be the most superficial anechoic chamber. Just deep to the AC is the lens, which is a hyperechoic concave structure. The retina is the innermost layer of the eye, which contains the photoreceptors. It is often not seen unless it is detached from the posterior aspect of the globe. The posterior chamber, which accounts for approximately 80% of the eye, will be the anechoic structure deep to the lens and is filled with vitreous fluid. Finally, the optic nerve is seen as a hypoechoic structure just deep to the retina ( Fig. 5.2 ).


Examination Technique
The structures of the eye are superficial and are best visualized with a high-frequency linear array probe. A transparent dressing can be placed on top of the closed eye for patient comfort and ease of cleaning the gel after the examination ( Fig. 5.3 ). Alternatively, clean gel may be placed directly on the closed eyelid. The patient should be placed in a supine to semi-upright position to maintain the gel’s position. Slightly cooling the gel will firm it and further maintain its position. To reduce theoretical iatrogenic injury, the ocular or eye examination setting should be chosen on the ultrasound machine. The examiner can maintain probe stability while scanning by placing the scanning hand on the patient’s nasal bridge, forehead, or cheek, and use copious amounts of gel to avoid transmitting unnecessary pressure to the eye. Limiting direct pressure on the eye is important for patient comfort, as well as reducing the oculocardiac reflex, which may decrease the patient’s heart rate. Use the conventional method of transducer orientation, directing the indicator toward the patient’s right in the transverse plane and cephalad in the sagittal plane. Careful methodical scanning of all eye segments in two planes is needed to evaluate pathology. In each orthogonal plane the patient should be asked to move the eyes in every direction to allow for full dynamic evaluation of the anterior and posterior chambers, as well as confirm extraocular muscle function. Contraindications include open ocular trauma, periorbital wounds, globe rupture, or retrobulbar hemorrhage.

Indications and Pathology
Acute Loss of Vision
Retinal Detachment.
Retinal detachment (RD) has a prevalence of 0.3%, and patients can present with the classic symptoms of sudden, painless loss of vision or a monocular visual field defect, often described as a curtain coming down. Some patients may experience flashers or floaters a few days or weeks before loss of vision. Prompt diagnosis can expedite an ophthalmologic consultation and prevent further vision loss. It is important to assess for a macular-sparing RD, as this will warrant emergent surgery. If the macula is involved, patients may complain of central vision loss, which will require surgery within 1 to 2 weeks. The utility of POCUS in diagnosing RD has been established by several prospective studies, and a 2016 retrospective study of 109 patients by Jacobsen et al. demonstrated a sensitivity and specificity for the use of POCUS to be 91% (95% CI [90–99]) and 96% (95% CI [89–99]), respectively.
The retina is normally anchored to the choroid of the optic nerve and anterolaterally at the ora serrata in the ciliary body. On ultrasound, a RD will appear as a thick, hyperechoic, mobile, membranous flap in the posterior chamber that appears lifted off the posterior surface and moves in conjunction with the patient’s eye movements, and is tethered to the optic nerve ( Fig. 5.4 ). The macula is usually seen temporally (lateral) to the optic nerve; thus if there is macular involvement, the retina will appear completely detached up to the optic nerve, resembling a hyperechoic letter “V” in the posterior chamber ( Fig. 5.5 ).


Posterior Vitreous Detachment.
A posterior vitreous detachment involves the separation of the vitreous from the underlying retina due to degeneration and/or shrinkage. It can present with similar complaints as an RD with floaters and/or flashes, but may also be insidious. There is typically no mention of a curtain coming down. Floaters are a sensation of gray or dark spots moving in the visual field, and flashes are typically described as monocular repeated flashes of white light in the peripheral visual field. This pathology is more common with increasing age, with a 24% prevalence in adults 50 to 59 years of age and reaching up to 87% in adults 80 to 89 years of age. Thus suspicion for this pathology should be higher on the differential diagnosis if an elderly patient presents with these symptoms. Other risk factors that may contribute are myopia, trauma, and intraocular infections. On dynamic ultrasound imaging, an undulating, echogenic membrane will be present in the posterior chamber, and it should move freely and swirl in the opposite direction that the eye is turning ( Fig. 5.6 ). Additionally, vitreoretinal adhesions can lead to retinal tears or avulsions of peripheral blood vessels, which may lead to vitreal hemorrhages. Unlike an RD, where the hyperechoic membrane is tethered to the choroid of the optic nerve, a vitreous detachment is free floating. Because a vitreous detachment does not typically lead to permanent vision loss, it is clinically significant to distinguish from an RD.
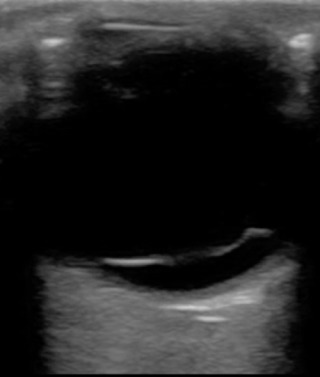
Vitreous Hemorrhage.
Neovascularization from dia-betic retinopathy, sickle cell retinopathy, and ocular ischemic syndrome is a vasoproliferative disorder that creates vessels prone to bleeding, leading to vitreous hemorrhage. Normal vessels can be torn in trauma or due to RD. A final source of hemorrhage can be blood from retinal microaneurysms or age-related macular degeneration. Blood in the posterior chamber causes obstruction of the retina during fundoscopic examination but does not impede the use of ultrasound. The patient may complain of loss of vision, floaters, red cape or hue, or “black rain.” The ultrasound characteristics depend on the severity of bleeding and the time course. Of note, the vitreous hemorrhage can also resemble an RD, as there will be echogenic, fibrous membranes floating in the posterior chamber. In the early stages, dynamic imaging can show very subtle low-amplitude echoes. As hemorrhage progresses, fibrinous vitreous echogenic membranes may appear and will be mobile at first, but may stiffen over time ( Fig. 5.7 ). To distinguish it from an RD, observe for any free movement of the echogenic material; this can be referred to as having a “snow globe” or “washing machine” pattern, and is not observed in RD. There will also be absence of any attachment to the optic disc. For hemorrhage associated with RD, urgent surgery is often needed. Hemorrhage without associated RD is treated based on the underlying cause and should be referred urgently to an ocular specialist, as laser therapy or vitrectomy may be needed. Asteroid hyalosis is another condition that may mimic vitreous hemorrhage. It may be present in up to 2% of the population and is caused by vitreal calcium–phosphate crystal deposits that appear as hyperechoic floaters in the posterior chamber. Most patients are asymptomatic, and very few require intervention.

Central Retinal Artery Occlusion.
Central retinal artery occlusion (CRAO) is another presentation of painless acute vision loss. Although there are multiple etiologies for CRAO, such as giant cell arteritis, emboli have an associated higher mortality due to cardiovascular risk factors. On POCUS, a long-standing embolus, whether from thrombotic material or cholesterol, will become calcified and demonstrate a “spot sign” if present at the distal aspect of the retinal artery adjacent to the optic nerve. This appears as a small, hyperechoic spot in the central retinal artery adjacent to the optic nerve. Using the color Doppler function allows examination for ocular blood flow in the ophthalmic and central retinal artery and vein. With this function, the examiner may notice a retrobulbar hyperechoic “plaque” suggestive of an embolus. Additionally, with use of the color Doppler, the examiner can see decreased, or even the absence of, blood flow within the central retinal artery.
Ocular Trauma
Lens Dislocations (Ectopia lentis).
Ectopia lentis, a lens dislocation or malposition, is largely due to trauma but can also be seen in patients with collagen disorders (Marfan’s and Ehlers-Danlos syndrome). Patients can present with a painful red eye, photosensitivity, diplopia, and partial or complete loss of vision. In a cross-sectional study by Ojaghi Haghighi et al., POCUS use by emergency medicine physicians was shown to have an 84.6% sensitivity (CI 95% [53.7–97.3]) and a 98.3% specificity (CI 95% [93.3–99.7]), respectively. There are two types of dislocations: partial (subluxation) and complete.
- •
With partial dislocation (subluxation), the lens will be partially attached to the ciliary body, and on dynamic ultrasound imaging can be seen as a convex hyperechoic structure partially attached to the ciliary body. Changing the patient’s position during imaging can aid in determining whether the patient presents with a complete or partial dislocation.
- •
With complete dislocation, the lens will appear completely in the vitreous, and on ultrasound imaging may even be overlying the retina ( Fig. 5.8 ).