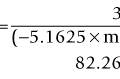Chapter 17 Ocular Laboratory Applications
Introduction
Utilization of high-frequency ultrasonography, also referred to as ultrasound biomicroscopy or ultrasound backscatter microscopy, to visualize microscopic scale structures began in the 1930s with the advent of acoustic microscopes and laser scanning microscopes.1–3 These instruments were designed to offer “acoustic” contrast in excised, thin tissue specimens, without the necessity for time-consuming cross-sectioning and staining procedures necessary in traditional histopathological examination. While these microscopes were not widely accepted as alternatives to optical microscopy, the use of high frequency, pulsed transducers for in vivo longitudinal, high resolution visualization of tissue began to emerge in the 1980s (Chapters 4 and 6).4,5 Providing greater depth of view than optical systems, these high frequency probes were initially utilized in clinical applications for ophthalmology and dermatology. Using a 100 MHz probe, Pavlin et al,6 were able to acquire images of Schlemm’s canal, cornea, iris, ciliary muscles, and retina in intact eye cross-sections at axial depths approaching 4 mm and with a lateral resolution of 20 µm. Similarly, 20–30 MHz probes have been utilized to accurately assess extent and thickness of skin melanomas and basal cell carcinomas in large patient cohorts.7,8
Instrumentation
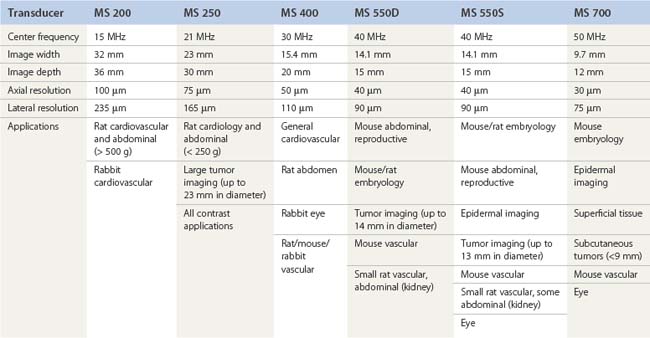
A pioneer in the development of the high frequency ultrasound transducers and ultrasound biomicroscopy, Dr. Stuart Foster founded VisualSonics in 1999 and developed the first commercial preclinical ultrasound. The most recent version of this system, the Vevo 2100, utilizes a 256-element linear array transducer that offers dynamic focusing, Doppler steering, acquisition rates of 300–400 frames per second, and axial resolutions approaching 30 µm (Figure 17.1A). Depending upon the application and size of the animal model utilized (i.e., zebrafish to rabbits), a user can select one of six transducers that range in frequency from 15 to 50 MHz (Table 17.1). Each transducer offers three adjustable focal depths and can acquire two modes simultaneously.
For imaging of larger organs or anatomical regions, transducers may be hand held; however, for applications that require strict transducer motion control such as image-guided injection, high-resolution imaging of small structures, or temporal imaging, transducers may be mounted onto the bench-top Vevo imaging station (Figure 17.1B). This “integrated rail system” provides a mount for free rotation and fixation of the transducer, an animal platform that sits above a ball-joint for precise angling, and a set of rails for X–Y positioning of this platform and Z-axis movement of the transducer mount. Animal platforms (dedicated rat or mouse) include imprinted sensors that monitor respiration, ECG, and heart rate, each of which are captured in real time and can be displayed/exported with every image series acquired (Figure 17.1C). Platforms can be heated to a user-defined temperature and a rectal probe is available to monitor animal temperature (particularly important for denuded mice prone to hypothermia). A nose-cone is attached to each platform for continuous flow of isoflurane supplied by an external anesthesia system (Figure 17.1D). For real-time, image-guided injection/extraction of drugs, contrast agents, genetic material, cells, and retroviruses an optional injection mount may be added to the Vevo imaging system. This injection mount includes a separate set of rails for positioning and can accommodate any number of syringes and needles.
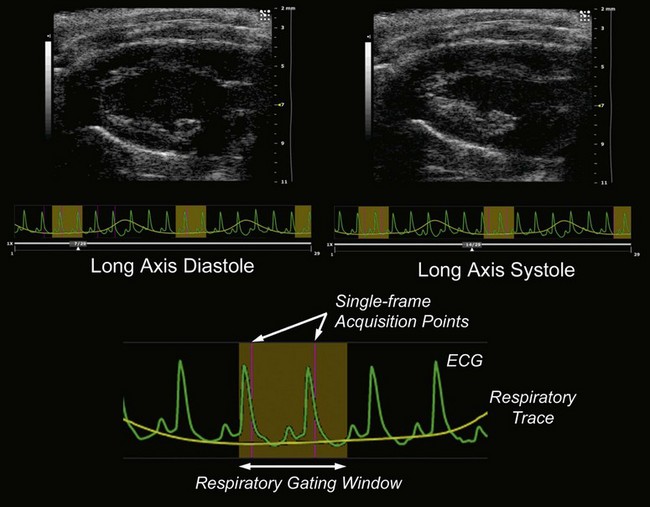
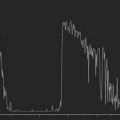
Due to the rapid heart-rate of small animals, particularly under anesthesia, cardiac and respiratory motion can significantly affect image quality and accuracy of subsequent measurements. To compensate, the respiration/ECG signals acquired from the animal platform described above, may be utilized to gate/trigger image acquisition. This provides users with the ability to make measurements and attribute phenomena to specific respiratory and cardiac phases (Figure 17.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree