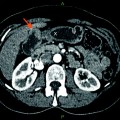
Fig. 1
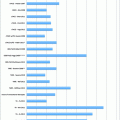
A Hilar cholangiocarcinoma with portal vein confluence encasement. a Abdominal CT showing the conduct of the mass with the portal vein bifurcation and the narrowing of the right anterior branch. b Percutaneous trans-hepatic cholangiogram detecting a IIIa tumor. c and d Intraoperative aspects before and after portal vein resection with end to end anastomosis
1.1.1 Hilar Cholangiocarcinoma
Hilar cholangiocarcinomas represent about 60 % of all treated biliary cancers, and aggressive surgery to achieve an R0 resection has long been recognized as the corn stone of therapy (Figs. 2, 3). Jarnagin et al. [3], in a review of the Memorial Sloan-Kettering Cancer Center (MSKCC) experience, from 1991 to 2000 identified 80 patients, 62 of which achieved an R0 resection. The overall survival was 42 months in the R0 group against 21 months in those with a positive microscopic margin (P < 0.0075). A similar association between positive margin of resection and survival was reported in 281 patients treated at Johns Hopkins between 1974 and 2004 [12].
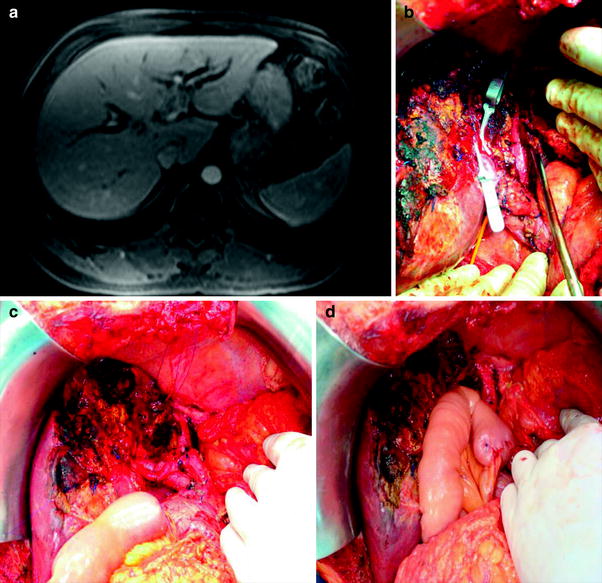
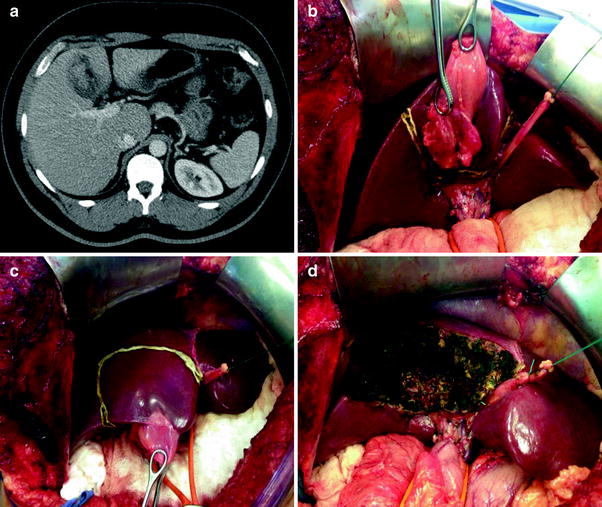

Fig. 2
A Hilar cholangiocarcinoma Bismuth–Corlette type IIIB treated by left lobectomy and caudate resection. a Abdominal CT showing the extension of the tumor to the left main duct. b–d Intraoperative view after liver transection with hanging liver approach, resection of the caudate lobe, and intrahepatic bilioenteric anastomosis

Fig. 3
Radiological and intraoperative aspects of gallbladder cancer. a Abdominal CT with a heterogeneous mass in the gallbladder fundus. b–d Radical cholecystectomy with en bloc resection of segments IVB and V and hilar lymphadenectomy
Lymph nodal involvement and number of lymph nodes evaluated also appeared to affect outcomes. In the experience of MSKCC, among patients with hilar malignancy treated with negative resection margins and no nodal involvement, but for whom fewer than 7 portal nodes were evaluated, had a worse survival relative to N0 patients who had >7 nodes evaluated [13].
Whereas vascular involvement has usually been considered a feature of unresectability, reports from Japanese hepatobiliary centers have revealed long-term survival subsequent to isolated portal vein resection [5, 14]. The results of hepatic artery resection have been more disappointing, and most studies recognize no long-term survivors among these patients. Nonetheless, one retrospective report from Nagoya demonstrated a survival advantage with combined portal vein and hepatic artery resection in patients with gross involvement of these vessels [5]. Among 50 treated patients, R0 resection was possible in 30 cases and the 5-year survival rate in this group was 40.7 % against 30.3 % in the entire cohort.
Patients with non-metastatic locally advanced and unresectable disease typically receive palliative chemotherapy and/or radiation therapy (RT). The Mayo clinic, however, has described more promising results with the use of orthotopic liver transplantation (OLT) in selected patients [15].
1.1.2 Intrahepatic Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (IHC) (Fig. 4) is a relatively uncommon disease, and surgical resection seems to be the only therapy associated with long-term survival. Recurrence following resection is, nevertheless, common. Interestingly, retrospective reports demonstrate conflicting result regarding the impact of R0 resection on survival [16, 17]. A review of 44 patients with IHC treated at Johns Hopkins from 1973 to 2004 showed that those with a negative resection margin had a statistically longer median disease-free and overall survival than those undergoing R1/R2 resection or palliative procedures [12]. A similar review at MSKCC from 1990 to 2006, though, demonstrated longer survival in patients treated by surgery (36 months vs. 9 months) but failed to demonstrate any benefit to R0 versus R1 resection [16]. These conflicts were probably due to the small number of patients in these series making them underpowered. In 2011, an international multi-institutional database reviewed data from 449 patients who underwent surgery for ICC between 1973 and 2010 and identified an unquestionable negative impact of positive resection margins on survival, with a hazard ratio of 2.20. Those authors also demonstrated that multinodular disease and vascular invasion, but not tumor size, were independent prognostic factors of survival in the absence of lymph node spread. In patients with N positive disease, who correspond to 30 % from those who had had a lymphadenectomy, these factors became the main prognostic factor of survival [18].
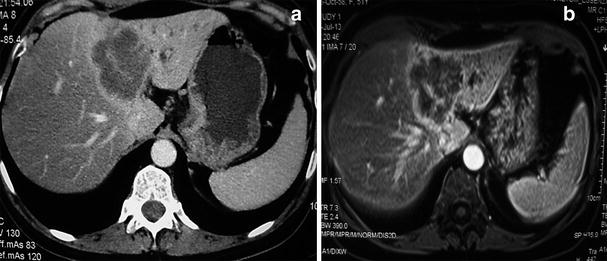

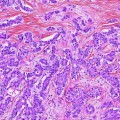
Fig. 4
Radiological aspects on CT and MRI of intrahepatic cholangiocarcinoma. Note the heterogeneous enhancement, left lobe atrophy and biliary dilatation
Despite the lack of prospective evidence, it seems that achieving a negative margin (R0) at the time of surgery offers a survival advantage over incomplete resection or chemotherapy alone, even if a major hepatic resection is required. For those patients who do experience recurrent disease, some authors have demonstrated that aggressive treatment of liver recurrences, including re-resections or ablation and further chemotherapy, may provide a survival benefit. A retrospective review from the University of Bologna studied 39 patients with recurrent disease treated between 1988 and 2008 and compared twenty-three cases treated with additional surgery and chemotherapy and 16 patients that received only systemic therapy. The three-year disease-free survival in the first group was 60 % against 0 % among patients with less aggressive approach [17]. The best evidence so far points out that all the efforts to obtain a R0 resection margin are justified in the primary setting and possibly for recurrence.
2 Surgical Results
2.1 Morbidity and Mortality
Due to the extensive surgery that is sometimes necessary that may include biliary and hepatic resections to obtain a R0 procedure, perioperative morbidity and mortality are noteworthy. In the treatment of gallbladder and biliary tract cancers, morbidity and mortality can reach rates of 14–76 and 0–19 %, respectively [19]. The most common complications following these operations are bleeding, biliary fistula, liver failure, and infections, including cholangitis, liver abscess, intra-abdominal abscess, wound infection, and pneumonia. They account for over 50 % of all adverse events [3, 20, 21]. Due to the high perioperative risk, the complexity of surgical procedures required and the rarity of these tumors, these patients should be managed only in tertiary cancer centers. Postoperative hepatic failure and its associated mortality have been associated with the extent of liver resection [22, 23], and various efforts have been employed in order to reduce its incidence.
2.2 Recurrence
Unfortunately, recurrence rates after resection of biliary tract cancer are high, reaching levels of 50–75 %. The most common sites of recurrence following resection include the hepatic pedicle, liver, and peritoneum [20, 24, 25]. Median disease-free survival ranges from 12 to 43 months. Prognostic factors for recurrence include histologic grade, pathologic T and N stage, and margin status [20, 24, 25]. Recently, a multi-institutional database analysis of 301 patients who underwent surgery for ICC pointed the liver as the most common site of recurrence, followed by the combination of intra- and extra-hepatic disease. Furthermore, factors associated with increased risk of recurrence were macrovascular invasion, nodal metastases, unknown nodal status, and tumor size [26]. Owing to the fact that the majority of patients with recurrent disease are not amenable to curative intent, progress in adjuvant therapy is necessary to improving long-term outcome.
2.3 Outcomes
The 5-year survival rates for these patients vary from 25 to 40 % in recent series [19]. Many clinic pathological factors have been shown to have an impact on long-term outcome, including negative histologic margin status, concomitant hepatic resection, absence of nodal involvement, lower AJCC T stage, well-differentiated tumor grade, papillary tumor morphology, and lack of perineural spread [3, 20, 22, 27]. Among these, a complete resection with histologically negative margins is the only modifiable factor and is therefore the primary goal of surgical therapy. Although some authors have identified a benefit of a R1 resection compared with no surgical therapy, a “planned” R1 procedure is still not recommended [20, 28, 29].
3 Emerging Strategies
3.1 Combined Treatment
Along with the better understanding of molecular characteristics and staging of gallbladder and biliary tract cancer, new multimodal treatment strategies to improve the survival have begun to emerge. Hopefully, with more effective systemic therapies, this will allow for more effective selection of patients with loco regional advanced disease suitable for subsequent surgical resection. However, up till now, the role of the combination of non-operative therapies and surgery is not clear.
In the setting of metastatic disease, treatment options include palliative chemotherapy with gemcitabine and platinum-based regimens. Recently, the addition of erlotinib to gemcitabine and oxaliplatin doublet has demonstrated an improved response rate, but no impact on progression-free survival [30]. Many groups have investigated the possibility of using targeted therapies, especially in patients with advanced IHC, but there is no definitive data on to support their use at the current time [31]. Typically, there is no role for surgery for patients with systemic recurrence of biliary tumors, with few cases reported in the literature of patients undergoing surgery for metastatic disease after tumor control with systemic treatment [32].
The liver is the main recurrence site of resected biliary tumors, often as multinodular spread. For IHC, whether this clinical presentation corresponds to primary tumor satellite lesions or foci of intrahepatic metastasis, or even multiple primary tumor foci, is sometimes impossible to determine [33]. Regardless, multi-focal disease—especially multi-focal recurrent disease—reflects an aggressive disease biology and a relative contraindication for surgery.
Furthermore, the disappointing results particularly in the setting of locally advanced disease and/or lymph node metastases have fostered the idea of neoadjuvant treatment, in order to increase the rate of complete resections and select the best candidates for surgical approach [34]. Although the literature is very scarce regarding this topic, the same rational has been used in other tumors with advanced disease and poor prognostic factors, including adenocarcinomas of the esophagus and pancreas, for example.
In a retrospective analysis, this approach did not show any benefit, leading to a possible delay in the surgical therapy, which may be associated with worse survival [35]. It is noteworthy that, in this series, the patients with the most advanced disease should have been referred for neoadjuvant treatment, which already biases this group to having a worse prognosis thereby perhaps explaining, in part, the inferior results in this group. The current recommendation is the surgical approach for all patients when resection with negative margins can be expected, as long as one can preserve adequate liver volume. The choice of preoperative systemic and loco regional therapy should be reserved only for unresectable cases, including chemotherapy and radiotherapy and/or intra-arterial chemotherapy.
The use of trans-arterial therapy for biliary tract tumors, especially IHCs, has been investigated both for unresectable tumors and in the adjuvant setting. The most frequently used technique consists in the administration of microspheres carried with chemotherapeutic agents, including doxorubicin and gemcitabine, either in an embolization procedure or as continuous infusion chemotherapy. The data are largely from Asian and demonstrate advantage in terms of response rate and progression-free survival in uncontrolled retrospective series for patients with locally advanced disease or metastases confined to the liver [36]. The lesion’s size, underlying liver function and tumor vasculature on imaging studies could predict the results obtained with this therapeutic modality.
After a R0 resection, the arterial chemoembolization was related to a longer relapse-free survival in patients with objective worse prognostic factors in a single series; however, it still needs stronger evidence [37].
Other treatment options for unresectable cases are radioembolization with Yttrium and photodynamic therapy, the latter with positive results in terms of progression-free survival in two small prospective and randomized studies [38].
Recently, a multi-institutional analyses from five major American centers compiled data of 198 patients with advanced ICC treated with intra-arterial therapies between 1992 and 2012. The majority of patients received conventional transarterial chemoembolization (cTACE–64.7 %), although 23.2 % had Yttrium-90 radioembolization. Complication rates demonstrated that this is a safe procedure and most patients experimented stable disease or partial response. There was no difference in survival rates regarding the type of intra-arterial therapy, and the results were better for those who had response on image exams.
The supporting data for the addition of systemic therapy and/or radiotherapy after surgical resection of these tumors are heterogeneous, as most of the studies are from single institutions and retrospective. The number of nodes, angio-vascular invasion, and lymph node metastases were identified as the main prognostic factors for overall and recurrence-free survival for IHC, with greater impact than other historical prognostic variables used previously for these neoplasms, as tumor size, for example. Such features should then guide the selection of patients for systemic treatment in research protocols.
The use of chemotherapy alone comes from the extrapolation of results from prospective studies for adjuvant treatment of pancreato-biliary cancer, with different proportions of patients with gallbladder and biliary tumors included in the study population [39]. Likewise, the use of radiation and chemotherapy after surgical resection is based on retrospective analyzes that demonstrated benefits in terms of progression-free and overall survival [40, 41].
3.2 Preoperative Management
3.2.1 Preoperative Biliary Drainage
The impact of preoperative biliary drainage on outcome is controversial [24, 42, 43]. Preoperative biliary drainage is associated with an increased risk of cholangitis, lengthened postoperative hospital stay, and may hamper the ability to determine the extent of the tumor during surgery. Conversely, unrelieved biliary obstruction is correlated with hepatic and renal dysfunction and coagulopathy [24, 44, 45].
Some authors attest that patients with hilar cholangiocarcinoma will benefit from biliary drainage of the anticipated remnant liver to enhance its capacity for post-resection hypertrophy. Because of potential difficulties in effective endoscopic stent insertion, and to optimally define the intrahepatic biliary anatomy, biliary drainage for hilar cholangiocarcinoma is often performed percutaneously. Described morbidity of percutaneous trans-hepatic catheter location includes the following: hemobilia, hepatic artery pseudoaneurysm, intrahepatic arteriovenous fistula, and catheter tract dissemination [46–48].
3.2.2 Portal Vein Embolization
Resection of >80 % of the total liver volume is correlated with major complications and prolonged hospital stay for patients with normal liver function [49, 50], and resection of >60 % of the total liver volume is associated with increased major complications, postoperative liver failure, and mortality in patients with compromised liver function secondary to chronic liver disease, chronic biliary obstruction, or high-dose chemotherapy [51]. Preoperative portal vein embolization (PVE) was first described in 1986 and is currently used to increase the volume and function of the future liver remnant (FLR) [52]. This strategy has been used prior to major hepatic resection for hilar cholangiocarcinoma, hepatocellular carcinoma, and hepatic resection of colorectal metastases [50].
Numerous studies have found that portal vein embolization accelerates hepatic mitochondrial function and induces hepatocyte proliferation in the non-embolized segments [53, 54]. The potential benefits of PVE are its capacity to induce hypertrophy in the FLR, thereby reducing the risk of postoperative liver failure, and its ability to permit curative resection for patients who otherwise would be considered unresectable due to insufficient FLR.
Prospective randomized trials and single institutional series support the safety and efficacy of preoperative PVE [55, 56]. A potential disadvantage of performing PVE is that it is sometimes difficult to determine preoperatively whether a right or left hemihepatectomy will be required if the tumor is located centrally at the hilus. Currently, there is no evidence to support routine use of PVE for hilar cholangiocarcinoma, but PVE should be considered for potentially resectable patients with normal liver function when anticipated FLR <20 % of the total liver volume, or patients with compromised liver function when anticipated FLR <40 % of the total liver volume. Most patients with hilar cholangiocarcinoma present with jaundice and are considered to have cholestasis-induced compromised liver function.
Recently, a German group [57] has introduced a rescue technique of accelerated liver regeneration to perform extended hepatectomy by right portal vein ligation combined with in situ splitting the liver. This new two-staged procedure, known as “Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS)”, may offer a new approach to treat patients with peri-hilar cholangiocarcinoma [58]. However, a call for caution has been made with the new ALPPS approach because of a high postoperative morbidity (70–90 %) and even mortality. Further data are necessary before ALPPS can be recommended for the treatment of patients with PHC [57–59].
3.3 Minimally Invasive Surgery
There is very little evidence in the literature regarding laparoscopy procedures to treat biliary tract cancer. Performing major laparoscopic hepatectomy remains a challenge among most hepatobiliar surgeons. The technical complexity and infrequent approach to this tumor and technique, along with the fact of unanswered questions about potential worse oncological results in good prognosis patients discourages most surgeons. Laparoscopic liver resection has become an acceptable and safe alternative to open procedure for the treatment of benign and selected malignant liver tumors, though. Recent studies show morbidity and mortality rates similar to conventional open procedures. Technical experience in liver surgery and laparoscopy are necessary for performing laparoscopic liver resections successfully. Lobectomies should only be accomplished laparoscopically after gaining experience with smaller laparoscopic liver resections.
Kazaryan et al. [60] published their 10 year experience from a center in Norway with laparoscopic liver resections for benign and malignant liver tumors. One hundred and thirteen patients underwent 121 procedures, the vast majority of the sample corresponding to liver metastases of colorectal adenocarcinoma; there were only two cases of laparoscopic resection for cholangiocarcinoma. The rate of conversion to laparotomy surgery was 3.4 %, with a mean operative time of 164 min (50–488) and estimated blood loss of 350 mL (<50–4,000). There were ten intraoperative complications (10 %) and 18 postoperative complications (12.6 %). Only one patient died (0.7 %). The mean hospital stay was 3 days (1–42), and the need for opioids for pain control was 1 day (0–11). Free margins of resection determined by pathological examination were successful in 140 of the 149 procedures performed (94 %).
Gumbs et al. [61] recently, in a review showing the experience of three centers with expertise in hepatobiliary laparoscopic surgery, analyzed all patients with tumors of the gallbladder, hilar, and IHC undergoing laparoscopic surgery with curative intent; they excluded patients with distal cholangiocarcinoma undergoing duodenopancreatectomy. Fifteen patients underwent laparoscopic surgery for gallbladder cancer, with the average number of dissected nodes being 4 (1–11) and all patients had an R0 resection. Only one case was converted to open procedure (7 %). No patients developed biliary fistula, percutaneous drainage, and/or passages of drains/stents in the biliary tract. There was local recurrence in one case, despite the free surgical margins, and distant recurrence another one after 20 months of follow-up.
Nine patients underwent hepatectomy for IHC, and all bilio-enteric anastomoses were performed laparoscopically. Two patients developed biliary leakage, one of them died after percutaneous biliary drainage due to intracavitary hemorrhage secondary to uncontrolled bleeding even after laparotomy. A third patient developed pulmonary thromboembolism. The morbidity rate was 33 and 11 % mortality. Only one case was converted to open surgery, and six patients remain alive after a mean follow-up of 22 months [61].
Five patients with hilar cholangiocarcinoma underwent minimally invasive liver resections, and two of them were in need of major liver resections. The estimated blood loss was 240 ml (0–400), and mean hospital stay was 15 days (11–21). All patients were alive after a median follow-up of 11 months; no recurrences were detected in any portal sites operated in 29 cases [61]. There is nothing significant to mention about robotic surgery for cholangiocarcinoma in the literature, except a few case reports so far.
3.4 Staging Laparoscopy
Despite exhaustive preoperative imaging studies, a significant proportion of patients is found to have unresectable disease at the time of laparotomy [3, 20]. Of the patients who are explored with curative intent only 40–50 % are ultimately resectable, which has motivated close evaluation of the role of staging laparoscopy for patients with hilar cholangiocarcinoma. The yield and accuracy of laparoscopy for patients with hilar cholangiocarcinoma are between 25 to 42 and 42 to 53 %, respectively [62]. Laparoscopic ultrasonography can also be used and has been shown to increase the yield by up to 17 % [63]. To use this technology selectively in patients with a higher likelihood of harboring occult metastases, the MSKCC staging system has been used to predict findings of occult metastases at laparoscopy. In patients with T2/T3 tumors, 36 % had occult metastases detected at laparoscopy versus 9 % in patients with T1 tumors (p = 0.02) [64], suggesting that laparoscopy should be used for patients with T2/T3 tumors.
3.5 Extension of Resection
3.5.1 Hepatic Resection
Over the past 20 years, there has been an increase in the use of hepatic resection in patients with hilar cholangiocarcinoma. Major hepatic resection addresses both direct hepatic invasion and intraductal extension of hilar cholangiocarcinoma to achieve negative radial and longitudinal resection margins. The inclusion of major hepatic resection as a fundamental surgical strategy for this disease has improved the proportion of R0 resections [3, 20, 22, 27], enhanced recurrence-free survival outcomes, and decreased the prevalence of hepatic recurrences [20]. Interestingly, many reports have shown improved survival with major hepatic resection even in the patients undergoing R0 resections, and poor outcome associated with extra-hepatic bile duct excision alone [3, 20, 22, 65].
However, there is still some debate about the impact of hepatic resection in survival of patients with Bismuth and Corlette type I or II tumors, specially type I. Ikeyama et al. [66] retrospectively assessed surgical outcome of 54 patients with Bismuth and Corlette type I and II hilar cholangiocarcinoma and showed survival benefit from right hepatectomy with caudate lobectomy for nodular and sclerosing tumors, but not for papillary tumors. Other group has reported no significant difference in survival between hepatectomy and bile duct resection alone for B–C type I and II tumors [67]. This needs to be further weighed in larger studies with longer follow-up, but for now, most experts agree that liver resection is necessary for hilar cholangiocarcinoma. Moreover, extra-hepatic bile duct resection was associated with a greater risk of positive resection margins and worse lymph node clearance in a recent multi-institutional series [68].
For T1a gallbladder tumors, there is no need for hepatic resection. However, to T1b, when the muscularis is invaded, most centers agree with that. In published series, the five-year survival rate for patients with T1b gallbladder cancer having undertaken radical resection averages 87.5 %, whereas it averages only 61.3 % in patients who have been submitted only to cholecystectomy [69]. A recent published decision analysis suggests that radical surgery for T1b tumor, just as stage II, is related to improved survival if compared with cholecystectomy alone [69]. Tumors greater than T1a, therefore, must be treated with standardized liver resection and lymphadenectomy. The hepatic resection must include segments V and IVb in most series. It is still controversial whether the anatomical resection of segments V and IVb is superior to gallbladder bed resection. In a recent analysis of a nationwide data from the Japanese Biliary Tract Cancer Registry, the data of 85 patients with pT2 gallbladder cancer patients were retrospectively compared: fifty-five treated with gallbladder bed resection, and 30 with S4a + 5 hepatectomy. The five-year survival rate did not differ significantly between the two groups. Recurrence occurred most frequently in both lobes than in S4a + 5 of the liver following gallbladder bed resection. They concluded that S4a + 5 hepatectomy was not superior to gallbladder bed resection alone for those cases [70].
In the case of direct invasion of right pedicle or deep liver invasion, an extended right trisectionectomy, in a fit patient with localized disease, must be performed. In this situation, also adjacent structures, as hepatic flexure of the colon, should be resected en bloc. Long-term survival has been reported from some centers, ranging from 15 to 63 %, with these extended procedures [71].
3.5.2 Caudate Lobe Resection
The caudate lobe ducts join the left and right hepatic ducts near their confluence, although the primary drainage is to the left hepatic duct [20, 72]. This intimate anatomical relationship explains the remark that the caudate lobe is involved by hilar cholangiocarcinoma in 40–98 % of patients [20, 72–74]. Retrospective studies have shown a decrease in local recurrence and improvement in five-year survival when concomitant caudate lobe resection is performed [24, 75, 76]. Routine caudate lobe resection, without direct invasion, however, remains controversial. Most institutions perform caudate lobe resection selectively, depending on tumor location, preferably with left-sided tumor.
3.5.3 Vascular resection
Portal vein resection and reconstruction have been performed for hilar cholangiocarcinoma with conflicting results [13, 22, 77, 78]. Although several retrospective series have shown no difference in operative mortality between patients undergoing portal vein resection and patients who did not [22, 77, 78], the influence of portal vein resection on long-term survival is less clear. Neuhaus et al. [22] proposed portal vein resection as part of the “no-touch” technique for the management of tumor and adjacent tissue. Portal vein resections were identified as an independent positive prognostic factor in their multivariate analysis of patients undergoing R0 resection, when initial 60-day mortality was excluded. However, overall 60-day mortality after portal vein resection was 17 % as compared with 5 % for patients without portal vein resection, and all of these deaths occurred after noncurative surgeries. Many other studies have shown equivalent or worse survival in patient undergoing en bloc resection of the portal vein [78–80]. Recently, de Jong et al. [68] identified 51 patients who underwent portal vein resection in an international multi-institutional database of 305 cases of hilar cholangiocarcinoma. In these series, PVR was most likely associated to a right-side hepatectomy and allowed a better lymph node clearance (median number of lymph node resected—6 vs. 4 in patients who underwent only bile duct and liver resection, p = 0.03). The incidence of R0 resection was not different between patients who had or not PVR, as well as the five-year survival rate. The 30-day mortality was, however, significantly higher in the group of PVR (6.7 vs. 11.8 %, p = 0.03). It is noteworthy that those rates were no longer significantly different when the 90-day mortality was analyzed. Those authors could also demonstrate that the survival rate in the group of patients who had disease that grossly involved the main portal vein and necessitate PVR was comparable with the five-year survival rate for the patients who underwent a “non-touch” technique, it means those who probably received a prophylactic PVR, as proposed by the German group. It is likely that the role of routine resection of the portal vein will not be directly defined unless a randomized clinical trial can be completed.
3.5.4 Pancreas resection
Distal tumors are often considered to be periampular tumors, as it is difficult to characterize their origins before resection. Most cases are treated with classical pancreaticoduodenectomies with or without pylorus preserving and standard lymphadenectomy [12, 81, 82]. During the past decade, more aggressive approaches, combining pancreaticoduodenectomy with portal vein resection, have gained wider appreciation. Pancreatic fistula is still the Achilles’ heel of this surgery and varies from 3 to 30 % depending on the expertise of the surgical team and the stiffness of the pancreas, whereas perioperative mortality is presently less than 5 % [82, 83]. The five-year survival rate is about 30 % [12, 84]. The most important prognostic factor is a negative resection margin [12, 83, 85].
3.5.5 Hepato-pancreato-duodenectomy
Major hepatectomy can sometimes be combined with pancreaticoduodenectomy in the presence of positive proximal bile duct margin when extending to only one hemiliver [86]. This procedure, sometimes labeled as hepatopancreatoduodenectomy (HPD), remains controversial and performed in only a few centers because of a high rate of mortality (15–60 %). Two recent series have shown that R0 resection was possible in between 73 and 85 % of the cases [87, 88]. The largest series, presented by Ebata et al. [88], reported on 26 cases of distal cholangiocarcinoma with increasing R0 resection after HPD and five-year patient survival rate of 37.5 %. The main drawback of such an approach was high morbidity of 77 %, but low mortality of 2.4 %.
Another recent study shows a similar experience, but with a higher mortality rate of 13 % [87]. The incidence of severe complications must be anticipated with this type of surgery, ranging from 31 to 100 % [86, 88]. In summary, this aggressive approach must be restricted to a few large-volume centers, and only for the purpose of a R0 resection. Perioperative mortality rates should ideally not exceed 5 %, and no longer be as high as 50 % as in historical series [89]. The approach warrants further investigation including the role of neoadjuvant or adjuvant chemotherapies.
3.6 Lymph Node Dissection
Metastasis to regional lymph nodes in hilar cholangiocarcinoma is common and is an important prognostic factor influencing survival after resection for hilar cholangiocarcinoma and gallbladder cancer [20, 90, 91




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree