Fig. 1
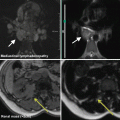
Mega-arachnoid cyst in the left frontobasal lobe in a 38-year-old patient detected during an MR scan performed for exclusion of metastases of an extracranial primary

Fig. 2
An incidental finding of minor significance, by means of a very small meningeoma in the right frontal lobe

Fig. 3
Liver MR scan performed for further characterization of a CT-detected lesion in liver segment 7 (haemangioma marked with arrow in a). Incidental finding of moderate significance in the same patient, by means of a gall stone in the common bile duct (arrow b)
In terms of classification of IFs, the majority of studies are consent on the graduation of IFs according to their clinical relevance, mainly into three to four categories. Most studies stratify the incidental findings into three categories as follows (Teuber et al. 2016):
Category 1: Normal findings/Incidental finding without clinical significance, including anatomical variations within the normal range (cavum septi pellucidi), known pathologies or (common) findings without prognostic relevance (e.g., developmental venous anomalies)
Category 2: Incidental finding that requires further radiological or medical evaluation, for exampe, additional sequences or contrast-enhanced examinations (suspected neoplastic lesions)
Category 3: Incidental findings that require immediate medical referral (space-occupying lesion, suspected acute hemorrhagic stroke)
Some classification system put further emphasis on the timing of referral according to clinical relevance (Katzman et al. 1999):
Category 1: No referral necessary, normal or findings common in asymptomatic subjects (e.g., sinusitis)
Category 2: Routine referral; findings not requiring immediate or urgent medical evaluation, but should be reported to the referring physician (e.g., old infarction)
Category 3: Urgent referral required within weeks of study for any abnormality that will need further yet nonemergent evaluation (e.g., low-grade astrocytoma)
Category 4: Immediate referral required (e.g., subacute subdural hematoma)
The type of disclosure of the IF to the participant depends on its clinical relevance, differentiating between direct (phone) contact to the participant within a 24 h period in case of urgent IFs and a standardized letter within 10 days for reportable, yet not actionable IFs (Bamberg et al. 2015).
Body imaging
Similar to brain imaging, there is no universal classification system for incidental findings in body imaging either, leaving the dedicated classification of the IFs to study-based guidelines and ethical standards. Nevertheless, similar to brain imaging, there is a universal consent on graduation of the incidental findings according to their clinical relevance. One rather general classification system, that is, recommended by the Royal College of Radiologists, subdivides the common IFs on body imaging into three major categories according to their potential implications for medical management (The Royal College of Radiologists 2011):
Category 1: Major significance – always requiring further investigation and likely to have adverse health effects (e.g., aortic aneurysm >5 cm, aortic dissection, solid liver mass)
Category 2: Moderate significance – usually requires further investigation but health effects unclear; (e.g., gallstone in common bile duct (Fig. 3), splenomegaly, indeterminate liver lesion)
Category 3: Minor significance – rarely requires further investigation and unlikely to have adverse health effects (e.g., left-sided inferior vena cava, gallstones in gallbladder).
While this general classification system covers a majority of the most common IFs on body imaging, it provides rather little guidance on IF management, in terms of timing and type (letter, phone call) of disclosure of the IFs to the participants. Hence, to ensure correct IF and disclosure handling, most population-based studies on body imaging provide more detailed IF management guidelines.
In the National German cohort study, an expert panel categorized potential incidental findings into three groups, comprising “actionable,” “reportable,” and “nonreportable” IFs in accordance with clinical guidelines, recent research results and ethical considerations.
- 1.
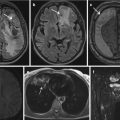
Actionable results are defined as incidental findings that bear a high likelihood to affect the participants’ well-being within a short time and require urgent medical treatment. This group of IFs comprises, for example, pneumothoraces, aortic dissection. After detecting and reporting the IF, the reader is required to seek for direct participant contact and further guidance of clinical work-up (Fig. 4).

Fig. 4
Urinary congestion of the right kidney in a participant of a population-based cohort study. Immediate IF disclosure to the participant is required
- 2.
Reportable results involve findings that are associated with a reasonably high likelihood to alter the participants’ well-being, such as aortic aneurysms with a diameter >5 cm or an abdominal mass >3 cm. In case of a “reportable result,” the participant is informed via standardized letter within a time period of <10 working days.
- 3.
All other IFs are categorized as nonreportable results without known clinical relevance, including renal cysts, gall bladder stones, etc. (Fig. 5) (Bamberg et al. 2015).

Fig. 5
Nonreportable IFs in two different participants of a population-based cohort study. The arrows mark a liver cyst (a) and bilateral parapelvin renal cysts (b)
3 Classification of Incidental Findings in a Clinical Setting
Within the last decades, imaging itself, and particularly cross-sectional imaging, has evolved to become an inevitable part of patient management, including assessment of acute and chronic benign diseases as well as staging, therapy monitoring, and aftercare of malignant diseases. While the aim of imaging in the clinical setting is set to address to sought the reason the study was ordered, the growing number of imaging examinations, particularly cross-sectional scans performed per patient, results in an increasing number of incidental findings. While IF classification and management is fairly settled in a research setting due to imposed study-based guidelines, the management of IFs detected in clinical imaging is not guided by clear-cut recommendations, causing high variations in practice among reporting radiologists. An important difference between IFs detected in the clinical setting and IFs detected in a research environment, which may significantly influence patient management and is also reflected in most IF recommendations, is caused by the readers’ knowledge of patient history, previous imaging studies, and potential comorbidities. Furthermore, in contrary to the predominantly MR-based research imaging studies, CT imaging plays an important role in clinical patient care, imposing a platform for other types of incidental findings that may not be detected by MRI, such as subsolid pulmonary nodules or atherosclerotic calcifications. In a systematic review by Lumbreras et al., the mean frequency of incidental findings was found to be as high as 23.6 % with an increased frequency of IFs in studies involving CT technology (mean 31.1 %) (Lumbreras et al. 2010). In a publication by Barrett et al., the reviewers analyzed the prevalence of incidental findings in trauma patients detected by computed tomography imaging, classifying the incidental findings into two categories: type 1 findings comprise findings that are potentially serious results and that demand further evaluation and close follow-up; type 2 findings comprise findings that require informing the patient but do not necessitate further follow-up. A third group of IFs comprise findings of little clinical consequence and did not necessitate patient notice, such as sinus mucuous retention cysts (Barrett et al. 2009). The analysis revealed a significant number of trauma patients diagnosed with potentially serious incidental findings, including 32.0 % of type 1 findings and 51.2 % of type 2 findings with the female sex showing a higher association to type 1 findings. While abdominal atherosclerosis (9.0 %), pulmonary nodules (7.4 %), and thoracic/mediastinal lymphadenopathy (5.6 %) constituted the most frequent type 1 IFs, a total of 631 incidental findings were considered suspicious of neoplastic foci (Barrett et al. 2009). Renal cysts, interstitial lung diseases, hepatic cysts, diverticulosis /-it is, and fatty liver were stated among the top five type 2 IFs, requiring patient information, yet no further follow-up investigations as proposed by the study protocol.
Numerous guidelines, mostly dedicated to organ-specific lesions such as the Fleischner classification for pulmonary nodules (Fig. 6), have been published over the years (MacMahon et al. 2005; Naidich et al. 2013). To provide a more comprehensive overview and management guidance, the American College of Radiology released conjoint recommendations, comprising pulmonary and abdominopelvic IFs as well as vascular findings (Berland et al. 2010; Heller et al. 2013; Khosa et al. 2013; Patel et al. 2013; Sebastian et al. 2013), including solid and subsolid pulmonary nodules, adrenal lesions, pancreatic cystic lesions, liver and renal lesions, splenic lesions, lymph nodes, as well as IFs of the biliary tract.


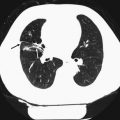
Fig. 6
Two pulmonary nodules (<4 mm) detected in a 52-year-old patient with no history of smoking or other risk factors. According to the Fleischner criteria no follow-up is needed
Examplatory organ-specific classification systems will be shown in the following section.
Lung
Incidental pulmonary nodules are encountered commonly in chest radiography and even more so in cross-sectional imaging due to its higher resolution and improved lesion-to-lung contrast. The incidental detection rates have been noted as low as 0.09– 0.2 % of all chest radiographs (Albert and Russel 2009) and as high as 31 %, for example, in a cohort study of patients undergoing CT scans for coronary calcium scoring (Burt et al. 2008). Overall, the estimated prevalence of solitary pulmonary nodules in the literature ranges from 8 to 51 % (Albert and Russel 2009). A solitary pulmonary nodule (SPN) is defined as a well-circumscribed, radiographic opacity measuring less than or equal to 30 mm in diameter, surrounded completely by aerated lung, and is not associated with adenopathy or atelectasis (Albert and Russel 2009; Gould et al. 2007). The differential diagnosis for pulmonary nodules comprises benign and malignant causes and demands further correlation regarding its radiologic features, patient history, as well as patient risk factors for cancer. Radiographic criteria utilized to estimate the probability of malignancy of a pulmonary nodule comprise potential calcification, nodule size, growth rate, as well as edge characteristics (Gurney et al. 1993; Cummings et al. 1986). While a lesion size <5 mm, smooth borders, solid density, and concentric or popcorn-like calcifications are considered suggestive for benign SPN, a lesion size >10 mm, spiculated borders, as well as a doubling time ranging from 1 month to 1 year are considered suggestive for malignancy (Albert and Russel 2009). Out of the above-named radiologic features, the size of the lesion seems to show the strongest link to the probability of malignancy at the time of detection as the prevalence of malignancy is 0–1 % for nodules <5 mm, 6–28 % for nodules 5–10 mm, and 64–82 % for nodules >20 mm in diameter (Wahidi et al. 2007). For nodules more than 3 cm in diameter, 93–97 % are malignant (Siegelman et al. 1986). After careful consideration of all clinical and radiographic criteria and estimation of probability of malignancy, further patient management regarding future (noninvasive) surveillance or potential invasive evaluation should be performed in accordance with the guidelines. A widely applied guideline for management of pulmonary nodules was introduced by the Fleischner society, categorizing solid and subsolid pulmonary nodules according to their size and patients’ risk for malignancy and recommending follow-up imaging or PET/biopsy, accordingly (MacMahon et al. 2005; Naidich et al. 2013).
Kidney
With renal cysts being one of the most common incidental findings in abdominal imaging, renal lesions detected on CT imaging are categorized into solid and cystic lesions, including a more dedicated classification of the cystic lesions according to Bosniak (Berland et al. 2010). The Bosniak classification is a well-accepted means of triaging renal incidentalomas, subdividing renal cysts into five groups according to their morphologic features (Curry et al. 2000):
Category 1: Benign simple cyst with thin wall without septa, calcifications, or solid components; no contrast-enhancement, water-equal density.
Category 2: Benign cyst with a few thin septa, which may contain fine calcifications or small segments of mildly thickened calcification. This includes homogenous, high-attenuation lesions less than 3 cm with sharp margins but without enhancement. Hyperdense cysts must be exophytic with at least 75 % of its wall outside the kidney to allow for appropriate assessment of margins, otherwise they are categorized as IFs.
Category 2F: Up to 5 % of these cysts are malignant and as such they require follow-up imaging, though there is no consensus recommendation on the appropriate interval of follow-up. Well-marginated cysts with a number of thin septa, with or without mild enhancement or thickening of septa. Calcifications may be present; these may be thick and nodular. There are no enhancing soft tissue components. This also includes nonenhancing high-attenuation lesions that are completely contained within the kidney and are 3 cm or larger.
Category 3: Indeterminate cystic masses with thickened irregular septa with enhancement.
Category 4: Malignant cystic masses with all the characteristics of category III lesions as well as enhancing soft tissue components independent of but adjacent to the septa.
With increasing likelihood of malignancy, category 2F cysts show a risk of malignancy of up to 5 %, category 3 cysts of 50 %, and the majority of category 4 cysts are shown to be malignant, affecting patient management regarding follow-up and/or surgical procedures accordingly.
Adrenal gland
Adrenal incidentalomas are considered a disease of modern technology, as their detection as an incidental finding has significantly increased with improving technology and increasing application of cross-sectional imaging. The prevalence of adrenal incidentalomas has been reported as high as 8 % in autopsy series and 4 % in diagnostic imaging (Kapoor et al. 2008). Adrenal lesions can be categorized as primary or metastatic, benign or malignant, and functioning or nonfunctioning (Boland et al. 2008). Based on the significant association between the size of an adrenal incidentaloma and its likelihood of malignancy, adrenal masses are subdivided into two groups, by means of 1–4 cm in adrenal mass size and >4 cm. As approximately 70 % in adrenal masses >4 cm (85 % if larger than 6 cm) are known to be malignant, interventional investigations (biopsy/resection) are recommended accordingly (Berland et al. 2010). With nonfunctioning adrenal adenomas being the most common type of adrenal incidentaloma, recommendations on diagnostic procedures include CT densitometry and/or MR-based chemical shift imaging to detect a potential signal drop in Opposed-Phase-imaging, indicative for fatty adrenal tissue in adenomas (Boland et al. 2008). Recent recommendations also propose CT perfusion imaging to assess the washout kinetics of the adrenal lesions for further characterization (Boland 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree