Fig. 1
Sample page from the SHIP information brochure on the explanation of the meaning of MRI findings (page 16)
A video of the examination procedure was shown in the waiting area to all potential participants at the SHIP examination center, with the intent of familiarizing participants with the upcoming experience of a whole-body MRI, which requires the placement of coils along the entire body (Fig. 2). The video was recorded after repeated problems in the early phase of the study with participants feeling uncomfortable with and thus unable to handle the unexpected situation of being strapped up along their entire body.


Fig. 2
Picture of a SHIP test volunteer being fully embedded by the coils before the start of the whole-body MRI examination
Immediately before the MRI examination, a research radiologist personally described the whole-body MRI examination and the process of handling incidental findings to the study volunteer again and provided the opportunity to clarify any concerns regarding the examination. The participant provided his written final consent to take part in the MRI examination at this occasion.
It is important to note that almost 400 SHIP-2 and SHIP-Trend participants refused to take part in the MRI examination after expressing their initial willingness, indicating that in-depth information may change the attitude toward participation, potentially because of the high burden of the examination to the participant.
All participants could opt-out in written from receiving disclosure of incidental findings. This option was only chosen by two participants, while all others requested the commutation of findings. This reflects the genuine interest of most participants in knowing more about their health. There was one important limitation to the opt-out option. If a severe finding posed a serious potential threat of damage to a third party, its disclosure had to be accepted by the potential participant, and rejection would have resulted in the exclusion of a participant from the MRI examination. This did not happen.
Although we made extensive efforts to properly inform our study volunteers about the limitations of research MRI, therapeutic misconception could not be avoided (Erdmann et al. 2011; Schmidt et al. 2013). Nearly all participants (97 %) expected to find out whether they were healthy or not. This was surprising given the fact that we stated the opposite in written and oral form on several occasions before and after the examination. Furthermore, 22 % of males and 8 % of females believed that they no longer needed to participate in recommended routine screening examinations. Almost half stated that they sought to learn more about a pre-existing physical complaint. It seems that the demand for more information about one’s own health is a key motivational factor for participation in a health study. This is implied by other studies as well (Kirschen et al. 2006).
4 Assessment and Handling of Incidental Findings in SHIP
The whole-body MRI implementation in SHIP was the first of its kind in a large general population cohort. The interpretation of findings was complicated for several reasons. First, the low pretest probability of serious pathologies (Volzke et al. 2012; Royal and Peterson 2008) in a general population sample likely reduces the positive predictive value of any finding. Second, the entire context of supporting clinical findings to aid in diagnosis is missing. Therefore an elaborated procedure was adopted to decide on the categorization and disclosure of MR findings. A primary goal was to protect participants from harms due to false-positive findings and from findings without forseeable therapeutic benefits. The assessment of incidental findings was conducted in two stages and has been described in detail elsewhere (Hegenscheid et al. 2013).
The procedure of assessing incidental findings began while the participant was present at the MRI unit. At that time, an ad hoc reading of the scans was performed by a trained radiologist to identify one of nine predefined life-threatening conditions requiring immediate referral, including acute brain infarctions, intracranial hemorrhage, or pneumonia. If present, these were disclosed to the participant immediately on site after the end of the MRI scan, and, if possible, the participant was referred to receive further diagnostics and treatment within the hospital.
Next, a comprehensive reading according to a standardized protocol that included a total of 670 items for whole-body MRI was conducted after the examination. At least two trained radiologists reviewed all scans independently. A three-point scale was used to rate the overall image quality (good, moderate, or poor) and artifacts (none, mild, or major). Additionally, readers evaluated images for the presence or absence of pathological findings and anatomical variants. All clinical judgements were exclusively made on the basis of MR images and performed using a digital picture archiving and communication system (IMPACS ES 5.2, Agfa HealthCare, Mortsel, Belgium). In case of a difference between the two first readings, a third reading was conducted by a senior radiologist to reach a consensus (Hegenscheid et al. 2013).
Incidental findings were classified and handled according to a standardized protocol approved by the institutional review board. Findings were classified into three categories for this purpose (Hegenscheid et al. 2013):
- 1.
Category I findings were normal or common in asymptomatic subjects, e.g., anatomical variants, old brain infarcts, and sinusitis. This also included abnormalities without well-defined diagnostic and therapeutic consequences according to existing guidelines and best practice recommendations (e.g., disc herniation).
- 2.
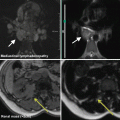
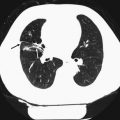
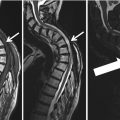
Category II findings were abnormalities needing further medical evaluation. Category II findings were disclosed to the participants by postal mail. Figure 3 lists selected precedents for Category II findings.

Fig. 3
Management protocol for the handling of incidental findings in SHIP. (1) Ad hoc reading with direct feedback to the participant in case of an acute finding requiring immediate referral (Category III). (2) Findings of potential clinical relevance were presented monthly to an interdisciplinary advisory board. The board subsequently recommend further clinical work-up (Category II) or not (Category I). (3) For frequent Category II incidental findings, the advisory board established precedents that were directly communicated to the participant
- 3.
Category III findings required immediate referral. A list of nine potential category III findings was defined in advance of the study (Fig. 3).
Only findings requiring further medical attention were eligible for disclosure. Category I findings were consequently not disclosed to the participants. All detected Category II and III findings were passed on to an interdisciplinary advisory board. This board was established before the study began. Its permanent members comprised specialists from medical, surgical, neurological, epidemiological, and radiological departments. Depending on the detected abnormality, additional specialists were invited. Upon presentation of findings they reached a consensus about whether or not to recommend disclosure. If the board decided against disclosure the finding was reclassified to Category I. There was one exception to this procedure: for frequent findings of clinical relevance, a list of precedents was established to aid further decision-making. This list contained conditions such as lung nodules >4 mm, renal cysts (Bosniak ≥ 2), and others (Fig. 3). Any finding corresponding to a precedent could be handled without further involvement of the advisory board.
The entire reading and decision process, including the communication of findings by a postal letter to the study volunteer, was supposed to be completed within 6 weeks. The letter comprised a short description of the finding and specific recommendations for further diagnostic and clinical work-up. All notified participants received the option to contact research radiologists by phone for assistance in case of any questions or concerns. This option was used only by a very small minority.
The decision to use letters was mainly based on logistic considerations as disclosure in person was considered to require too many resources. Category II or III findings were not sent directly to treating doctors to respect the study volunteer’s autonomy in the handling of their study findings. In addition, the feasibility of sending findings to doctors seemed low in the German medical system, where patients freely choose physicians of different specialities.
While a study might adopt a restrictive policy on the communication of findings, there are limitations. Study participants in Germany are entitled to receive all findings if they request them. In SHIP, MR images were not released to the participants routinely. However, study participants repeatedly requested them after the examination, and they were thus released to the participants.
5 Distribution of Incidental Findings in SHIP
Results from SHIP provide several important insights on the distribution and nature of findings in a general population cohort (Hegenscheid et al. 2013):
Get Clinical Tree app for offline access

- 1.
Incidental findings are very common.
Among the first 2500 study volunteers, 13,455 findings and anatomical variants of any category were documented either on the plain whole-body MRI or the contrast-enhanced modules.
- 2.
Most incidental findings are likely without medical importance.
In total, 12,125 (90.1 %) of all findings belonged to Category I.
- 3.
Severe findings requiring immediate medical attention are very rare. Only nine Category III findings among the first 2500 volunteers resulted in immediate medical action, some of which are shown in Fig. 4.