System
Number of criteria
Number of organs
Modality
Phase
WHO (1979)
28
9
Chemo
Acute
CTC (1983)
18
13
Chemo
Acute
RTOG/EORTC-Acute (1984)
14
13
RT
Acute
RTOG/EORTC-Late (1984)
68
17
RT
Late
LENT/SOMA (1995)
140
13
RT
Late
CTC v2.0 (1998)
152
22
RT
Late
CTCAE v3.0 (2003)
260
22
Alla
Acute
370
All
All
Acute and late
The radiation oncology profession has traditionally been concerned with reporting late effects of cancer treatment and the RTOG in conjunction with EORTC introduced both the “Acute and Late Radiation Morbidity Scoring Criteria” simultaneously (Cox et al. 1995). A series of NCI sponsored workshops led to the introduction of more comprehensive system entitled: LENT ~ Late Effects Normal Tissue and SOMA criteria, representing subjective Symptoms, Objective findings and MAnagement features. The A referred to Analytic quantifiable parameters in the laboratory or imaging. With acceptance and joint publications on both sides of the Atlantic, RTOG/EORTC hope to standardize reporting of late effects. (Trotti 2002; Rubin et al. 1995a) Some of the guiding thoughts to reduce interobserver variability was to replace the commonly used four grades of 1 + mild, 2 + moderate, 3 + severe, 4 + life threatening with better descriptors with corresponding terms as occasional, intermittent, persistent and refractory, respectively, when referring to the expression of symptoms and signs, i.e., pain. Longitudinal clinical trials emphasizing correlation of symptoms and sign of toxicity with metrics and interventions are future goals. (Trotti and Rubin 2003).
The most recent collaboration sponsored by all modalities has resulted in a more comprehensive CTC v3.0, which includes more late effects criteria and is inclusive of all modalities (Available at: http://ctep.info.nih.gov/CTC3/ctc.htm. Accessed 1 April 2003; Trotti et al. 2003). However, the merging of late effect and acute effect criteria, although more comprehensive with 510 criteria, when specifying anatomic sites or other subclassifications, raises the number to 900 adverse effect criteria for grading. The need for a summary toxicity methodology and a global adverse effect score, inclusive of multiple organ systems, has yet to be defined, and is essential for outcomes reporting.
2 The Biologic Basis For Combining Acute and Late Criteria
The most prominent feature of CTCAE v3.0 is the merging of early and late effects criteria into a single uniform document and the development of criteria applicable to all modalities. The research support for the concept of a “biologic continuum” is based upon the original paradigm by Rubin and Casarett (Rubin and Casarett 1968) in which the clinical radiation pathophysiologic course of events incorporating the dynamic sequence of cellular events and tissue specific effects began at the moment of radiation exposure. The schema illustrated radiation effects, both the clinical and subclinical events, in each organ system, but noted that depending on its cell population and tissue organization would express radiation syndromes differently. The underlying pathophysiologic commonality was the obliteration of the normal tissues’ fine microvasculature, whereas the time to clinical expression, the latent period, is related to stem cell depletion in either rapid or slow renewal system, i.e., acute versus chronic or early versus late effects. This paradigm was the first formalism linking acute and late effects as both a pathophysiologic and a clinical bio continuum. More recently, the molecular biologic events captured as a persistent cytokine cascade induced by radiation in a murine model has recapitulated the shape of the Rubin and Casarett tissue effect over time curves adding further to their validity (Rubin et al. 1995b). The arbitrary 90 day rule dividing early and late is no longer acceptable, since modalities overlap and are administered concurrently, and adjuvant chemotherapy is repeatedly cycled often for months and years. The use of a complex concurrent or hybrid sequential schedules undermines the usefulness of a simplistic temporally defined “early-late” construct. Moreover, there is a growing recognition that surgery (Fedyk et al. 2001; Mercado et al. 2002) and chemotherapy (Petak and Houghton 2001) much like radiation lead to molecular events resulting in a perpetual cytokine, chemokine cascade and surgery induces wound healing responses that result in a inflammation, fibrogenesis, and neoangiogenesis leading to epithelial regeneration. This multimodal molecular cascade leads to and supports the biologic continuum model (Fig. 1).
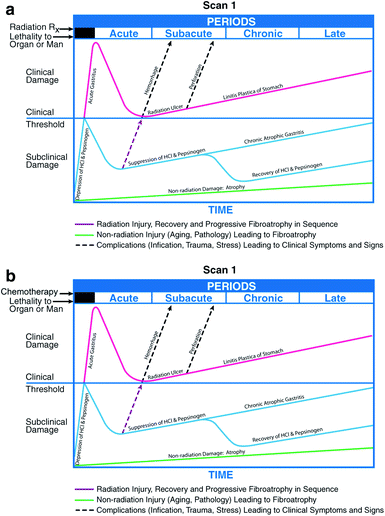

Fig. 1
The clinicopathologic course of events following irradiation can be complicated by the addition of chemotherapy. Similarly, chemotherapy can result in parallel set of events. a Classically, when radiation therapy precedes chemotherapy, the introduction of the second mode can lead to expression of subclinical damage or, when injury is present, to death. b The same is true if chemotherapy precedes radiation therapy. Reprinted with permission. (With permissions from Rubin & Casarett, 1968)
3 Standardization, and Statistical Reporting
Standardization of language requires use of the International Dictionary of Medical Terminology and commonly used disease codes, i.e., ICD 10 (World Health Organization 1992; International Statistical Classification of Diseases and Related Health Problems. 10th ed. American Psychiatric Publishing 1992) need to synchronized with both CTC and LENT-SOMA diagnoses. Thus, the descriptors of adverse effects language can become more uniform and will reduce interinvestigator variability. The introduction of quality of life scales to represent the patients’ viewpoint is an important aspect of grading adverse effects. Another important aspect is the need to integrate CTC and LENT-SOMA more fully. The LENT-SOMA is based on anatomic terms consisting of 15–20 major systems with approximately 50–60 subsites and is compatible but not identical with the terminology of the TNM system (American Joint Committee on Cancer (AJCC) 2002). By contrast, the CTCAE v3.0 utilizes more physiologic and functional terms and clinical syndromes. There is as much concurrence and similarities as differences and a comparison of terms is presented in Table 2. The anatomical terminology reconciliation of the 3 systems is consistent with the International Anatomical Terminology (Terminologic Anatomica) approved in 1998 by the International Federation of the Association of Anatomists (Table 2). (Federative Committee on Anatomical Terminology (FCAT) 1998).
Table 2
Anatomic-physiologic systems: hybrid nomenclature
Anatomic sites AJCCa TNM | LENT/SOMAb | Physiologic systems CTC v3.0c |
|---|---|---|
Central nervous system | (2) | Neurology |
(Neuroendocrinec) | (3) | Endocrine |
Ophthalmologic sites | (6) | Ocular/Visual |
Head and neck sites | (10) | Upper respiratory |
Digestive system | (6) | Gastrointestinal |
Major digestive glands | (5) | Hepato/Biliary/Pancreas |
Thorax breast | (1) | |
Lung | (1) | Pulmonary |
Pleura | (1) | |
(Heartc) | (1) | Cardiac, Arrhythmia |
(Vascularc) | (1) | Vascular |
Genitourinary sites | (7) | Renal/Genitourinary |
Male sexual reproduction | ||
Gynecologic sites | (7) | Female sexual reproduction |
Musculoskeletal | (2) | Musculoskeletal |
Skin | (1) | Dermatology, Lymphatics |
Lymphoid sites | (4) | Allergy, Immunology |
Bone marrow | (1) | Blood, Bone marrow |
Hemorrhage, bleeding | ||
Infection, coagulation |
There is a large and growing literature assessing both the CTC systems and LENT-SOMA. Numerous clinical trials have been published often comparing these systems with other late toxicity grading criteria, particularly in Europe. The literature is equally divided between concordance and discordance in confirmation of their applicability. The majority of reports are retrospective and not prospectively designed to assess validation, especially for LENT-SOMA. Davidson et al. (2002, 2003a, b); Routledge et al. (2003); Hoeller et al. (2003); Fehlauer et al. (2003); Denis et al. (2003); Anacak et al. (2001); Tawfiq et al. (2000) However, more recently, direct comparison has been made utilizing CTC v3.0 and LENT-SOMA. Furthermore, recent analysis of a validation perspective clinical trial in variety of anatomic sites by RTOG confirms that LENT-SOMA is a superior instrument as to capturing late effects. Utilizing a technique of linguistic analysis there are 12 recurrent criteria that apply to grading most of the organ systems. The “shared” word descriptors for each grade which can be identified in both LENT-SOMA and CTC v3.0 allows for a “concise grading dictionary” of well defined lexicons which captures the essence of both systems. The SOMAtization of CTC v3.0 is shown in Table 3, which provides a more focused selection of criteria and should enable users to record toxicities more efficiently and accurately. The array of criteria relate to five categories: symptoms, physical findings, interventions to ameliorate, quality of life, or activities of daily living. Laboratory values and imaging studies are working in progress as to correlations with gradations of toxicity and at this time should not override the other criteria when assigning grade.
Table 3
Somatization of CTCAE v3.0
Mild grade 1+ | Moderate grade 2+ | Severe grade 3+ | Life threatening grade 4+ | |
|---|---|---|---|---|
S | Asymptomatic | Symptomatic usually | Persistent symptoms | Refractory symptoms |
Minimal symptoms | Marked symptoms | Intensive symptoms | Symptoms unresponsive to medication | |
O | Transient signs | Intermittent signs | Symptoms apparent | Advanced persistent signs |
Functionally intact | Function altered | Function impaired | Function collapsed | |
M | No interventions |