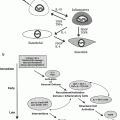
Fig. 1
Potential radiation biologic targets for translational research. For each of the pathways detailed molecular and cellular knowledge is continuously becoming available that explains cellular phenotype and provides novel therapeutic targets (Modified from Coleman CN. Harris JR: Current Scientific issues related to clinical radiation oncology. Radiat Res 1998:150:125.)
Table 1
Application of molecular biologic concepts to radiation therapy
|
Process
|
Potential manipulation
|
Examples of therapya
|
|---|---|---|
|
DNA damage
|
Increase damage in tumor cells
|
Hypoxic-cell sensitizers, thymidine analogues
|
|
DNA repair
|
Decrease repair in tumor cells
|
Fluoropyrimidines, hydroxyurea, cisplatin
|
|
Signal transduction
|
Inhibit protective signalling cascades in tumor cells
|
Protein kinase C inhibitors (?) phosphotyrosine kinase inhibitors(?)
|
|
Radiation-induced gene expression
|
Use gene therapy
|
Tumour necrosis factor linked to radiation responsive promotor(?)
|
|
Growth factor expression
|
Administer or increase expression of protective factors
|
Interleukin-1, granulocyte colony-stimulating factor granulocyte—macrophage colony-stimulating factor, basic fibroblast growth factor (?)
|
|
Block expression of factors producing long-term toxicity
|
Antibodies or antisense RNA against epidermal growth factor, transforming growth factor β (?)
|
|
|
Apoptosis
|
Force tumor cells to undergo apoptosis
|
Transfection of wild-type TP53(?)
|
|
Cell cycle
|
Synchronize tumor cells in sensitive phase of cycle (early S or M phase)
|
Antimetabolites (early S phase), paclitaxel phase (M), cyclin inhibitors(G, to S phase)
|
|
Prevent G2 arrest in tumor cells
|
Cyclin inhibitors(G2 to M phase)(?)
|
2.2 Cell Types According to Mitotic Frequency
In his classification of body cells on the basis of the kinds of individual lives they lead, Cowdry (1950) named four general classes: vegetative intermitotic, differentiating intermitotic, reverting postmitotic, and fixed postmitotic (Fig. 2a, b). Table 2 outlines the properties of these classes of cells and gives examples.
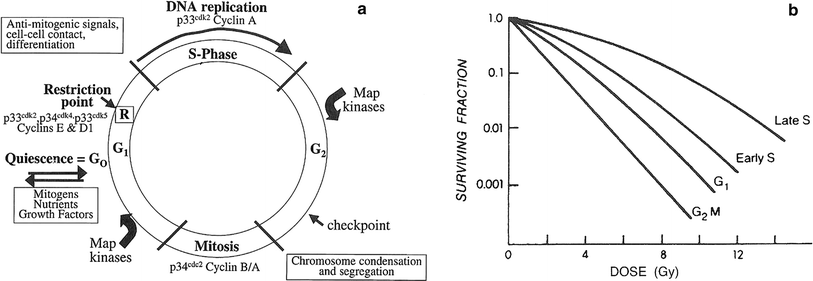

Fig. 2
a Cell cycle progression, with the major regulatory kinases indicated. Once a cell passes the restriction point (R), it is committed to progress through S-phase, even in the absence of mitogenic signals checkpoints (indicated in G2) have also been identified in G1, S1 and mitosis. b Patterns of radiosensitivity change through the cell cycle. This change is known as the “age function,” and varies widely among different cell types. Cells in mitosis are almost always sensitive, followed by cells at the G1/S boundary, whereas the period of greatest resistance is at late S. G gaps; G 1 first gap before DNA synthesis; G 2 M Second gap before mitosis; S DNA synthesis phase (Adapted from Sinclair WK: Cyclic X-ray responses in mammalian cells in vitro. Radiat Res 1968; 33:620, with permission. Copyright © 1967 OPA (Overseas Publishers Association) N.V.)
Table 2
Kinds of cell lives
|
Vegetative intermitotics
|
Differentiating intermitotics
|
Reverting postmitotics
|
Fixed postmitotics
|
|
|---|---|---|---|---|
|
Life span
|
Begins at mitosis
Ends at mitosis
Duration short
|
Same. Duration short, perhaps even shorter
|
Begins at mitosis
Ordinarily ends at death. When demand for more cells is great, ends at mitosis. Duration longer
|
Begins at mitosis
Ends at death. Duration still longer; for nerve cells very long, very short for neutrophilic leucocytes
|
|
Differentiation
|
Least. Constitutes reservoir of cells. Aging minimal
|
More as steps in acquisition of specific functions. Aging slight
|
Still more. Functionally mature. Aging marked
|
Most. Functionally mature. Aging greatest, ending in death
|
|
Service
|
As cell producers
|
As cells learning how to work
|
As cell specialists
|
As cell specialists even after death. Erythrocytes, epidermal cells, corneal cells, and ganoblasts
|
|
Examples
|
Basal epidermal cells, spermatogonia of testicle, hemocytoblasts of bone marrow
|
Spinous cells of epidermis, spermatocytes, erythroblasts, myelocytes.
|
Renal epithelial cells, smooth muscle cells, hepatic epithelial cells.
|
Nerve cells. Neutrophilic leucocytes
|
|
Principal locations
|
In stratified epithelia nearer to arterial bloodstream.
|
Sometimes farther away as in spinous layer of epidermis
|
In liver and adrenals nearest the arterial bloodstream
|
Almost anywhere needed
|
|
Malignant transformation
|
Occasional
|
Occasional
|
Rare
|
After youth, never
|
2.2.1 Vegetative Intermitotic Cells
These cells divide relatively frequently, have relatively short individual lives between divisions, differentiate little or not at all during their individual lives between divisions, show minimal aging changes, and function chiefly as producers of cells to replace themselves and the more differentiated cells of relatively short life span which die or are regularly lost from the body. The division of cells of this type produces daughter cells, some of which differentiate and others of which remain vegetative intermitotic cells.
2.2.2 Differentiating Intermitotic Cells
These cells also divide relatively frequently; have relatively short individual lives between divisions and show only slight aging changes. However, they undergo steps in the process of differentiation between divisions. Each individual differentiation intermitotic cell becomes increasingly more differentiated between divisions until it becomes fully capable of performing its specialized function, at which time it becomes either a reverting or a fixed postmitotic cell. The number of successive divisions in a line of differentiating intermitotic cells before the completion of differentiation varies with the type of specialized cell in question. For the sake of simplicity, only one row of differentiating intermitotic cells is represented in the diagram. Actually there may be several divisions, as in the production of mature blood cells in bone marrow, or there may be omission of this stage in other tissues, as in thin epidermis.
2.2.3 Reverting Postmitotic Cells
Reverting postmitotic cells are highly differentiated, functionally mature cells with relatively long individual lives which show marked aging changes and may die without producing daughter cells. These cells do not normally undergo regular or frequent divisions, but when the demand for more cells of their kind is great, e.g., after pathologic destruction, some of them may “revert” for a time to an intermitotic state, in that they divide exuberantly to replace cells lost.
2.2.4 Fixed Postmitotic Cells
Fixed postmitotic cells are very highly differentiated, functionally mature cells which have lost completely their ability to divide, regardless of functional demand. They show marked aging changes ending in death. Some cell types of this class, such as neurons, have long lives and die without replacement, whereas others, such as neutrophilic leukocytes, are relatively short-lived but are replaced by a supply of precursors produced by the divisions of vegetative and differentiating intermitotic cells and sometimes by the division of reverting postmitotic cells.
2.3 Classification of Cells According to Relative Radiosensitivity
The relative radiosensitivity of cells according to the kinds of lives they lead is illustrated in Fig. 3. This classification is given in the order of decreasing relative radiosensitivity and is based upon the criterion of cell death.
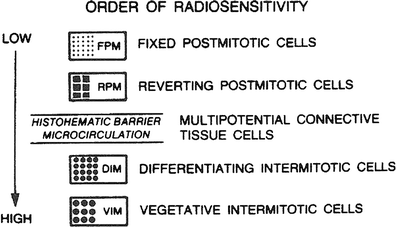

Fig. 3
Relative cell radiosensitivity. (From Rubin P, Casarett GW: Clinical Radiation Pathology. Philadelphia, W.B. Saunders, 1968, with permission.)
Radiation acts at molecular and cellular levels, and tissue effects represent a summation of those effects. Because the key target cells for the survival of a complex organ depends on the organization of all its tissues, cellular damage in one key cell population may result in death of the whole tissue. For example, small blood vessels are fairly sensitive to irradiation, so the effects on a tissue from disruption of its blood supply may be greater than those from the irradiation of the parenchymal cells themselves.
A relative cell radiosensitivity is illustrated in Fig. 3, which is based on the classification schema related to cellular division and differentiation by Cowdry. The uncommitted stem cell is a vegetative intermitotic cell; the committed stem cell is a differentiating intermitotic cell. Reverting postmitotic cells have the ability to divide when conditioned or challenged, such as hepatocytes after liver resection. The organization of tissues and organs by these cell types determines their radiosensitivity, which is based on their most radiosensitive cells. Table 3 reviews cell and tissue radiosensitivity.
Table 3
Classification of cells according to relative radiosensitivity
|
Tissues
|
Relative radiosensitivity
|
Chief mechanisms of hypoplasia
|
|---|---|---|
|
Lymphoid, hemopoietic (marrow), spermatogenic epithelium, ovarian follicular epithelium, intestinal epithelium
|
High
|
Destruction of vegetative or differentiating tissue cells of high mitotic frequency
|
|
Oropharyngeal stratified epithelium, epidermal epithelium, hair follicle epithelium, sebaceous gland epithelium, urinary bladder epithelium, esophageal epithelium, optic lens epithelium, gastric gland epithelium, ureteral epithelium
|
Fairly high
|
Destruction of vegetative or differentiating tissue cells of fairly high mitotic frequency
|
|
Ordinary interstitial connective tissue, neurological tissue (connective tissue of nervous system), finely vasculature, growing cartilage, or bone tissue
|
Medium
|
Damage or destruction of connective tissue cells of moderate mitotic frequency and damage to the fine vasculature
|
|
Mature cartilage or bone tissue, mucous or serous gland epithelium, salivary gland epithelium, sweat gland epithelium, nasopharyngeal simple epithelium, renal epithelium, pulmonary epithelium, renal epithelium, hepatic epithelium, pancreatic epithelium, pituitary epithelium, thyroid epithelium, adrenal epithelium
|
Fairly low
|
Hypoplasia secondary to damage to associated fine vasculature and connective tissue elements, with relatively less contribution by the direct effects on parenchymal cells, which normally divide infrequently
|
|
Neuronal tissue, muscle tissue
|
Low
|
Hypoplasia secondary to damage to associated fine vasculature and connective tissue elements, with little contribution by the direct effects on the parenchymal cells, which do not normally divide
|
2.3.1 Class 1: Vegetative Intermitotic Cells
These are generally the most radiation sensitive of cells. They are short-lived as individual cells, they are primitive, and they normally divide regularly to produce daughter cells, some of which will enter into a process of differentiation and others of which will not differentiate, but remain vegetative intermitotic cells. This class includes cells such as free stem cells of the hematopoietic tissues, e.g., hemocytoblasts, primitive lymphoblasts, primitive erythroblasts, and primitive myeloblasts; the dividing of cells deep in the intestinal glands (crypts of Lieberkuhn); the primitive Type A spermatogonia in the seminiferous epithelium; granulosa cells of developing and mature ovarian follicles; basal germinal cells of the epidermis and germinal cells of the gastric glands and of holocrine glands such as the sebaceous glands. Large and medium-sized lymphocytes also belong to this class. Small lymphocytes are also highly radiosensitive, but they do not divide as small lymphocytes. They have been observed on occasion to divide after first increasing in size. Nevertheless, they are exceptional in this class, although they are among the most radiosensitive cell types.
2.3.2 Class 2: Differentiating Intermitotic Cells
These cells are relatively radiosensitive, but are generally somewhat less sensitive to radiation than vegetative intermitotic cells. They are also relatively short-lived as individuals and are produced by divisions of vegetative intermitotic cells. They normally divide from time to time for a limited number of divisions, and they differentiate to some degree between divisions. The more differentiated they become, the less sensitive to radiation they become. This class includes cells such as dividing differentiating cells of the hematopoietic series in the intermediate stages of differentiation in both granulocytic and erythrocytic series in bone marrow, the more differentiated spermatogonia (intermediate and Type B), and the spermatocytes in the seminiferous epithelium and ovocytes.
2.3.3 Class 3: Multipotential Connective Tissue Cells and Endothelial Cells
This class of cells is intermediate in position between relatively radiosensitive cells in classes 1 and 2 and the relatively radioresistant cells in classes 4 and 5. These cells may divide irregularly or sporadically in time and in response to a variety of special stimuli, and they are generally capable of undergoing transitions from one to another morphologic and functional form under the influence of various stimuli. Their life span as individuals with respect to one form of function may be highly variable, but under normal conditions it is on average longer than that of cells in classes 1 and 2. This class includes such cells as endothelial cells, especially those of the smaller vessels, which distinction would imply the existence of some indirect mechanisms as yet unknown, and fibroblasts and mesenchymal cells.
2.3.4 Class 4: Reverting Postmitotic Cells
In general this class of cells is relatively radiation resistant. These cells experience relatively long lives as individuals, do not normally undergo regular or periodic division in the adult and generally do not divide except under abnormal conditions presenting special stimuli. Under an appropriate stimulus, usually involving damage, destruction, or loss of considerable numbers of cells of their kind or of cells which they are capable of producing, they can revert to a condition in which they divide to produce more cells of their kind or they undergo heterotypic transformations to produce cells of certain other kinds.
Some of the cell types of this class are highly specialized in function, while others are not. Some are epithelial and some are connective tissue elements. This class includes cells such as the epithelial parenchymal cells and the duct cells in the salivary glands, liver, kidney, and pancreas; basal and parenchymal cells of merocrine glands such as the sweat glands and of endocrine glands such as the adrenal, thyroid, parathyroid, and pituitary glands; cells of interstitial gland tissue of the gonads; cells of corpora lutea; Sertoli cells; septal cells of the lung; fixed stem cells of various tissues, such as the reticulum cells in hematopoietic tissues and perhaps some smooth muscle cells. The cells in this class may show considerable aging changes, but on division their daughter cells show a relative rejuvenation, with the disappearance of aging changes.
2.3.5 Class 5: Fixed Postmitotic Cells
The cells in this class are generally the most radiation resistant of cells. They normally do not divide or have lost completely the ability to divide under any circumstances and are highly differentiated morphologically and highly specialized in function. Some of them have very long lives and others have relatively short lives, but all undergo progressive aging changes until death, If not killed prematurely. The short-lived fixed postmitotic cells, when lost, are replaced by the action of vegetative and/or differentiating intermitotic precursor cells. If the loss of precursor cells is also reverting postmitotic primitive fixed stem cells. This class includes cells such as the long-lived neurons and perhaps some muscle cells, which are not replaceable, and the short-lived polymorphonuclear granulocytes, erythrocytes, spermatids, spermatozoa, superficial epithelial cells of the alimentary tract and epithelial cells of the sebaceous glands, all of which are replaceable.
2.4 Normal Tissues and Their Relative Radiosensitivity
The histopathologic manifestations of radiation cellular death are pyknosis and karyolysis, swollen vacuolated cells with loss of staining capacity, and altered permeability, with eventual degeneration and phagocytosis. The events following exposure to radiation doses are determined, in part, by the radiosensitivity of the cells in the parenchymal compartment as well as related to the radiosensitivity of vascular stroma and its turnover rate. A rapid renewal system, illustrated in Fig. 4a, consists of vegetative intermitotic cells, differentiating intermitotic cells, and fixed postmitotic cells as found in the skin or the mucous membrane of the alimentary tract in the testes. The initial fractional doses of irradiation destroy the stem cell compartments (vegetative intermitotic cells and differentiating intermitotic cells) and reduce the production of cells that normally flow into the postmitotic compartment. The lining or mucous membrane thins and, as the dose increases, the connective tissue becomes edematous. With large doses, the parenchymal compartment may be lifted or sloughed as a result of the edema. The ability of the tissue to regenerate depends on the survival of stem cells (vegetative intermitotic cells), which gradually increase in number, differentiate, and rebuild the postmitotic compartment. The compartments eventually stabilize, but they might be relatively reduced as a result of increased fibrosis and an increased histo-hematic barrier. If large doses have been given, the microcirculation might become occluded at a later time, leading to frank delayed necrosis. With lesser degrees of fibrosis, the parenchymal compartment might atrophy, and when stressed, as by infection, might show its limited stem cell reserve capacity or mitotic potential to respond.
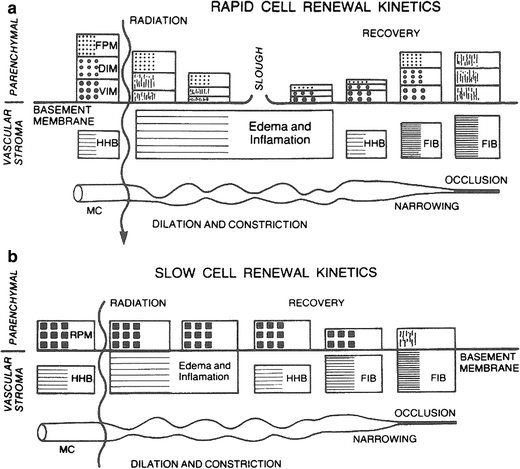

Fig. 4
a Rapid renewal system illustrating radiation effects on both parenchyma and micro vasculature compartments. VIM vegetative intermitotic cells; DIM differentiating intermitotic cells; FPM fixed postmitotic cells; HHB histo-hernatic barrier; FIB increased fibrosis; MC microcirculation (From Rubin P. Casarett GW: Clinical Radiation Pathology. Philadelphia, W.B. Saunders, 1968, with permission). b Slow renewal system illustrating effects on the vascular compartment, which leads to a late effect in the parenchymal cells as the capillary sclerosis and fibrosis increases. RPM reverting postmitotic cells (From Rubin P. Casarett GW: Clinical Radiation Pathology. Philadelphia, W.B. Saunders, 1968, with permission.)
The sequence of events differs in a slow renewal system or nonrenewal system (Fig. 4b). The parenchymal compartment consists of reverting postmitotic cells or fixed postmitotic cells. Little or no change occurs in the parenchymal compartment with the fractional dose schemes used clinically. The vascular stromal compartment more often determines the course of events, although there are effects that can be attributed to a direct effect on parenchymal cells. The late expression of injury of these cells is caused by their slow renewal, hence the slow expression of injury.
2.4.1 Concept and Criteria of Radiosensitivity of Tissues
As in the case of the cell, the radiosensitivity or radioresistance of tissues, relates to differences in degree of response to similar doses to differences in doses required to produce similar responses. These terms are relative designations and depend upon the criterion or effect used as a basis for classification. The criterion of relative radiosensitivity of tissue types used here is the relatively direct loss (hypoplasia) of the parenchymal (definitive) cells of the tissue, with resulting tissue atrophy. The term “relatively direct” is defined as the result of mechanisms entirely contained within the tissue specified.
2.4.2 Range and Basis of Radiosensitivity of Tissue Types
The mechanisms of relatively direct radiation-induced hypoplasia and atrophy of rapidly and continually self-repopulating tissues, i.e., tissues which contain the frequently dividing intermitotic cells, may chiefly include temporary or permanent inhibition of mitosis, normal and precocious maturation and loss of cells without replacement for a time, and much mitosis-linked death of cells. Since these tissues are self-repopulating from vegetative and/or differentiating intermitotic cells, they are highly radiosensitive during mitosis. These cellular effects require relatively small doses, such tissues being highly radiosensitive.
In the case of in frequently or slowly repopulating tissues, i.e., those in which the only source of repopulation are the rarely dividing, long-lived reverting postmitotic cells, the chief and almost the only mechanism of relatively direct radiation-induced hypoplasia and atrophy is interphase death, which requires large doses. Therefore such tissues are relatively radioresistant.
In the case of tissues which cannot repopulate their parenchymal cells, i.e., tissues containing irreplaceable, long-lived fixed postmitotic cells, the only mechanism of relatively direct radiation-induced hypoplasia and atrophy is interphase death, and therefore such tissues are extremely radioresistant.
As the dose is gradually increased in the irradiation of an organ, more and more indirect effects at the intertissue level are brought to bear on the irradiated tissues, reducing the degree of selectivity that is associated with relatively direct effects on the tissue. In the case of the relatively resistant, slowly repopulating tissues or nonrepopulating tissues, the hypoplasia and atrophy which may be caused by large doses is often due in large measure to relatively indirect (intertissue) mechanisms, prominent among which are effects mediated through damage reactions of the cellular elements of interstitial connective tissue and the fine vasculature. The microvasculature has a relative radiosensitivity which is intermediate between dividing intermitotic cells on the one hand and reverting or fixed postmitotic cells. Similarly, in the irradiation of organs or segments of the body, more remote indirect (interorgan or systemic) mechanisms may contribute to the total effect on tissues being irradiated.
The relative radiosensitivity of a tissue is determined largely by the relative radiosensitivity of its parenchymal cells. Therefore, the essential radiosensitivity of the fine vasculature and connective tissue cells is modified by the radiosensitivity of the different tissue types in which it is embedded. The radiation sensitivity of the fine vasculature and interstitial connective tissue is generally less than that of tissues containing vegetative and/or differentiating intermitotic parenchymal cells, but is generally greater than that of tissues composed only of reverting or fixed postmitotic parenchymal cells. Consequently, in tissues of the former type (containing sensitive parenchymal cells), radiation can cause early or acute lesions (hypoplasia, atrophy) largely through relatively direct mechanisms at the tissue level, with relatively little mediation of the effect through damage to the fine vasculature and interstitial connective tissue. On the other hand, in tissues of the latter type (containing resistant parenchymal cells), the production of early or acute lesions requires larger doses and is usually associated with more marked mediation of the effect through damage to the fine vasculature and/or connective tissue elements and a relatively lesser contribution to the lesion from relatively direct mechanisms of tissue effect.
The radiation damage changes in the fine vasculature and interstitial connective tissue play a very important role in the production of chronic or delayed lesions in tissues of all kinds. The chronic and delayed radiation lesions are associated with nutritional and metabolic disturbances of the tissues.
2.4.3 Classification of Tissues
In keeping with the above considerations, various types of tissues are listed in Table 3 in the order of decreasing radiosensitivity, on the basis of the relatively direct radiation effect (hypoplasia) on the tissues specified; the chief mechanisms of the tissue effect are indicated. Similarly, in Table 4 are listed various organs in the order of decreasing radiosensitivity, on the basis of the relatively direct radiation effect (parenchymal hypoplasia), which would include effects due to inter tissue mechanisms within the organs, such as effects on parenchymal tissues mediated through damage to the local fine vasculature and connective tissue; here, too, the chief mechanisms of the organ effect are indicated.
Table 4
Normal tissues and their relative radiosensitivity
|
Organs
|
Relative radiosensitivity
|
Chief mechanisms of parenchymal hypoplasia
|
|---|---|---|
|
Lymphoid organs, bone marrow (and blood), testes, ovaries, intestines
|
High
|
Destruction of parenchymal cells, especially the vegetative or differentiating intermitotic cells that are precursors of the mature parenchymal cells
|
|
Skin and other organs with epidermoid linings (cornea, oral cavity, esophagus, rectum, vagina, uterine cervix, urinary bladder, ureters, etc.)
Optic lens, stomach
|
Fairly high
Fairly high
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree