Oncologic Imaging
Dominique Delbeke
William H. Martin
 |
Some scintigraphic techniques used for oncologic imaging—such as technetium 99-m (99mTc)-diphosphonate bone imaging for primary and metastatic disease, 99mTc–sulfur colloid liver/spleen imaging for hepatic masses, 99mTc-pertechnetate and iodine-123 (123I)-iodide for thyroid masses, and 99mTc-diethylene triamine pentaacetic acid (DTPA) and 99mTc-mercaptylacetyltriglycine (99mTc-MAG3) for renal masses—are discussed in other chapters. Some nuclear medicine techniques offer relatively high specificity for tumor in general or for specific types of tumors. 131I-iodide and 123I-iodide imaging for metastatic thyroid carcinoma offer high specificity in the proper clinical context and are addressed in Chapter 1. Other agents that offer relatively high specificity include 131I-metaidobenzoguanidine (MIBG) or 123I-metaiodibenguanidine for neuroendocrine tumors, 111In-octreotide for tumor expressing somatostatin receptor (SSR), and radiolabeled monoclonal antibodies for tumors expressing a specific antigen.
Gallium-67 (67Ga) citrate, thallium-201 (201Tl) chloride, 99mTc-sestamibi, and monoclonal antibodies have been used as an important complement to cross-sectional imaging but have been largely replaced by 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET), which became the imaging modality of choice for oncologic imaging.
18F-FDG Imaging
18F-FDG is an analog of glucose and behaves as a tracer of glucose metabolism. Therefore the distribution of 18F-FDG is not limited to malignant tissue. 18F-FDG enters into cells by the same transport mechanism as glucose and is then intracellularly phosphorylated by a hexokinase into 18F-FDG-6-phosphate (18F-FDG-6-P). In tissues with a low concentration of glucose-6–phosphatase—such as the brain, the myocardium, and most malignant cells—18F-FDG-6-P does not enter into further enzymatic pathways and accumulates intracellularly proportional to the glycolytic rate. Some tissues—such as liver, kidney, intestine, muscle, and some malignant cells—have varying degrees of glucose-6-phosphatase activity and do not accumulate18F-FDG-6-P to the same extent. To interpret 18F-FDG images, one must be familiar with the normal distribution of 18F-FDG, physiologic variations, and benign conditions associated with 18F-FDG accumulation (1,2).
The cortex of the brain uses glucose as its only substrate; therefore 18F-FDG accumulation is normally high. The myocardium, on the other hand, can use various substrates according to substrate availability and hormonal status. In the fasting state, the myocardium utilizes free fatty acids; but after a glucose load, it favors glucose. When the thorax is evaluated with 18F-FDG to assess the presence of malignant lesions, a 12-hour fast is recommended to avoid artifacts due to myocardial activity. For evaluation of coronary artery disease, a glucose load with or without insulin supplementation is usually given to promote myocardial uptake of 18F-FDG. Unlike glucose, 18F-FDG is excreted by the kidneys, and focal ureteral accumulation should not be mistaken for metastasis. Concentration of 18F-FDG in the renal collecting system may obscure the evaluation of that region. This can be minimized by maintaining good hydration and by the administration of loop diuretics. For adequate visualization of the pelvis, irrigation of the bladder via a urinary catheter can be useful.
At rest, skeletal muscle uptake of 18F-FDG is low, but after exercise, significant accumulation of 18F-FDG in selected muscle groups may be misleading. For example, in the evaluation of head and neck cancer, uptake in the muscles of mastication or laryngeal muscles may mimic metastases. Therefore it is important for the patient to avoid chewing or talking during the distribution phase following 18F-FDG injection. Hyperventilation may induce uptake in the diaphragm, and anxiety-induced muscle uptake is often seen in the trapezius and paraspinal muscles. Muscle relaxants such as benzodiazepines may be helpful in anxious patients.
Another source of misinterpretation is uptake in the gastrointestinal (GI) tract. There is usually uptake in the lymphoid tissue of Waldeyer’s ring, and prominent uptake in the cecum of many patients may also be related to abundant lymphoid tissue in the intestinal wall. The wall of the stomach is often faintly seen and can be used as an anatomic landmark; similarly, esophageal activity is often seen.
Physiologic thymic uptake may be present in children and in patients after chemotherapy. Its typical smooth, symmetrical “V” shape usually allows differentiation from residual lymphoma. Marked diffuse bone marrow uptake is often seen after chemotherapy, especially if colony-stimulating factors were administered concurrently, and may compromise evaluation of the marrow for malignant involvement. Marrow uptake returns to normal 4 to 6 weeks after completion of chemotherapy.
18F-FDG is taken up by activated macrophages at sites of inflammation, and the uptake may often be sufficient to resemble malignant lesions when there is granulomatous inflammation, as with tuberculosis, sarcoidosis, fungal infections, and aspergillosis.
In order to avoid misinterpretation of 18F-FDG images, it is critical to standardize the environment of the patient during the uptake period, to examine the patient for postoperative site, drainage tubes, stoma, etc., and to time 18F-FDG imaging appropriately after invasive procedures and therapies. Although 18F-FDG shares some of the limitations of other tumor imaging agents—such as 67Ga, 201Tl, and 99mTc-sestamibi—the relatively high ratio of tumor-to-nontumor activity observed in most malignant lesions accounts for the reported high sensitivity and specificity of 18F-FDG imaging and the intrinsic characteristics of PET imaging.
Evaluation of PET images can be performed visually or semiquantitatively using the standard uptake value (SUV) or the lesion-to-background ratio (L/B). The SUV is the activity in the lesion in microcuries per milliliter corrected for the patient’s weight and the dose of 18F-FDG administered. The SUV may be more accurate when measured relative to body surface area or lean body mass than body weight. Although semiquantitative assessment offers a more objective estimation of the metabolic activity of the lesions, visual analysis is adequate for clinical purposes in most instances. The average SUV of normal liver parenchyma and blood pool is approximately 2.0 and can be used as a visual reference. The SUV depends on accurate
calibration of the positron emission tomography (PET) system, accurate soft tissue attenuation correction, and reconstruction algorithms among other factors. With PET/CT, attenuation correction is performed using CT-transmission maps acquired just before acquisition of the emission data; this procedure can be compromised by imperfect registration of the transmission and emission scans due to patient motion. The SUV is also dependent on factors that are difficult to control in the clinical environment, such as the patient’s plasma glucose and insulin levels, fasting state, dose infiltration, recent physical activity, and uptake time of 18F-FDG. In addition, there is inter- and intraobserver variability in identification of the lesion and slice with highest uptake and in drawing contours around the regions of interest. Therefore comparison of SUVs between two studies in the same patients for assessing response to therapy requires rigorous quality control, especially if performed on different PET systems or using different protocols and variation between institutions (3,4).
calibration of the positron emission tomography (PET) system, accurate soft tissue attenuation correction, and reconstruction algorithms among other factors. With PET/CT, attenuation correction is performed using CT-transmission maps acquired just before acquisition of the emission data; this procedure can be compromised by imperfect registration of the transmission and emission scans due to patient motion. The SUV is also dependent on factors that are difficult to control in the clinical environment, such as the patient’s plasma glucose and insulin levels, fasting state, dose infiltration, recent physical activity, and uptake time of 18F-FDG. In addition, there is inter- and intraobserver variability in identification of the lesion and slice with highest uptake and in drawing contours around the regions of interest. Therefore comparison of SUVs between two studies in the same patients for assessing response to therapy requires rigorous quality control, especially if performed on different PET systems or using different protocols and variation between institutions (3,4).
Indications
It is well established that neoplastic cells demonstrate increased metabolic activity. This is due in part to an increased density of glucose transporter proteins and increased intracellular glycolytic enzyme levels. Although variations in uptake are known to exist among tumor types, elevated uptake of 18F-FDG has been demonstrated in most malignant primary tumors. Therefore the most common indications for 18F-FDG imaging are as follows:
Differentiation of benign from malignant lesions
Staging and restaging malignant lesions
Detection of malignant recurrence
Monitoring of response to therapy
Following are the specific instances in which 18F-FDG imaging is most useful:
Rising serum tumor markers in the absence of a known source
Equivocal lesions on conventional imaging
Differentiation of posttherapy changes from residual or recurrent tumor
Preoperative staging of patients contemplated for curative resection
Somatostatin Receptor (SSR) Imaging
Somatostatin is a peptide hormone widely distributed throughout the central nervous system (CNS) and GI tract, including the pancreas. It interacts with its target cells via specific cell-surface SSRs, of which there are five subtypes (5). A wide variety of neoplasms also express high-affinity SSRs, most importantly the neuroendocrine tumors of the amine uptake precursor decarboxylation group, such as pituitary adenomas, carcinoid tumors, pancreatic islet cell tumors, pheochromocytomas, paragangliomas, medullary carcinoma of the thyroid, and small cell carcinomas of the lung. Activated lymphocytes and some lymphomas may also express SSRs. The high density of the type II SSRs on tumor cells allows their visualization with 111In-labeled octreotide, a somatostatin analog that is cleared rapidly from the circulation by the kidneys and has relatively minor hepatic activity, thus allowing identification of hepatic metastases.
Following the intravenous injection of 6 mCi of 111In-octreotide, planar whole-body images are acquired using a medium-energy collimator at 4 and 24 hours postinjection. SPECT images are required to assess the liver for metastases, and SPECT of the pelvis or thorax is required if clinically suspicious. Bowel activity at 24 hours can usually be differentiated from tumor by the absence of activity in that location on the early images; 48-hour images may sometimes be necessary. Liver, spleen, kidney, bladder, and bowel activity is virtually always seen, and thyroid and gallbladder activity is often seen. One must be careful not to mistake gallbladder activity for hepatic metastasis. Interfering activity may be seen in the lungs following radiotherapy and bleomycin therapy as well as with concurrent viral or bacterial infections.
The presence of SSRs on tumor cells correlates well with the success of pharmacologic nonradioactive somatostatin therapy. In imaging of growth hormone–secreting tumors, it is often advised to judge prospectively the potential success of parenteral octreotide therapy for effective growth hormone suppression and tumor regression. 111In-octreotide scintigraphy may be used to successfully differentiate adenocarcinoma of the pancreas from an islet cell tumor, and SSR imaging is the most accurate modality for the detection of these typically small islet cell neoplasms. Owing to the frequent absence of type II receptors on the cells of insulinomas, the sensitivity of SSR scintigraphy for the detection of insulinoma is only 50%. The success rate in the detection of paraganglioma and metastatic pheochromocytoma is high, and SSR scintigraphy is likely the most sensitive and specific indicator of carcinoid tumor extent. Various other tumors—such as medullary carcinoma of the thyroid, Merkel cell tumors, small cell lung cancer (SCLC), and lymphomas—have been imaged successfully. In general, 111In-octreotide imaging is preferable to MIBG scintigraphy in neuroendocrine tumors with the exception of benign pheochromocytoma.
131I- and 123I-Meta- iodobenzylguanidine (MIBG) Imaging
MIBG is a guanethidine analog that acts as a norepinephrine analog localizing in pheochromocytoma and paraganglioma via the normal norepinephrine uptake mechanism (6). The agent can be radiolabeled with 131I or 123I, and tumor uptake is not inhibited by adrenergic blocking drugs other than labetalol. 123I-MIBG is commercially available outside the United States, whereas 131I-MIBG is used in the United States to image patients with suspected pheochromocytoma and paraganglioma as well as a number of other neuroendocrine neoplasms. Because of the undesirable dosimetry associated with 131I, the injected dose is restricted to 0.5 to 1.0 mCi, yielding a poor-count image unsuitable for SPECT imaging. 123I-MIBG
imaging results in improved resolution, less noise, and statistics suitable for SPECT imaging without the 48- to 72-hour delay necessary for 131I-MIBG. Physiologic liver, spleen, bladder, and salivary gland activity is routinely observed, but distinct normal adrenal tissue is only rarely identified except when using 123I-MIBG. This technique is not used for screening but more for localization of a tumor suspected to be present due to elevated catecholamine levels. Since pheochromocytoma is bilateral in 10%, extra-adrenal in 10%, and malignant in at least 10% of patients, routine preoperative imaging with 131I-MIBG has been recommended and should be the first step in the evaluation of patients suspected to have recurrent or metastatic pheochromocytoma/paraganglioma. Owing to relatively poor sensitivity, radiolabeled MIBG is not used clinically to image medullary carcinoma of the thyroid and carcinoid tumors. Conventional imaging techniques are highly accurate in the detection of the primary focus of neuroblastoma and most metastases, but MIBG scintigraphy exhibits unique benefits in detecting skeletal metastases and differentiating a posttreatment scarring/healing mass versus persistent, viable tumor. Sensitivity for the detection of metastases is 88%, but it rises to 98% with SPECT utilizing 123I-MIBG.
imaging results in improved resolution, less noise, and statistics suitable for SPECT imaging without the 48- to 72-hour delay necessary for 131I-MIBG. Physiologic liver, spleen, bladder, and salivary gland activity is routinely observed, but distinct normal adrenal tissue is only rarely identified except when using 123I-MIBG. This technique is not used for screening but more for localization of a tumor suspected to be present due to elevated catecholamine levels. Since pheochromocytoma is bilateral in 10%, extra-adrenal in 10%, and malignant in at least 10% of patients, routine preoperative imaging with 131I-MIBG has been recommended and should be the first step in the evaluation of patients suspected to have recurrent or metastatic pheochromocytoma/paraganglioma. Owing to relatively poor sensitivity, radiolabeled MIBG is not used clinically to image medullary carcinoma of the thyroid and carcinoid tumors. Conventional imaging techniques are highly accurate in the detection of the primary focus of neuroblastoma and most metastases, but MIBG scintigraphy exhibits unique benefits in detecting skeletal metastases and differentiating a posttreatment scarring/healing mass versus persistent, viable tumor. Sensitivity for the detection of metastases is 88%, but it rises to 98% with SPECT utilizing 123I-MIBG.
Monoclonal Antibody Imaging
With the advent of hybridoma cell culture technology in the 1970s, it became possible to produce large quantities of homogenous murine monoclonal antibodies to a specific antigen expressed by tumor cells (7). The ideal antigen should be specific for one or several malignancies and be consistently expressed in high density in every patient with tumor and in every tumor deposit in each patient. However, because the extent of antigen expression is correlated with the degree of tumor cell differentiation, antigen expression for a specific tumor and even for a specific patient at different sites or times is heterogenous or mosaic. Furthermore, most tumor-associated antigens are not truly specific and are often expressed by various benign tissues, albeit at a lower density.
The first two monoclonal antibodies to become commercially available in the United States were (a) satumomab pendetide (OncoScint), an 111In-labeled whole murine IgG antibody directed against an antigen, TAG 72, expressed by 83% of colon and 97% of ovarian carcinomas, and (b) nofetumomab (Verluma), a 99mTc-labeled murine Fab-fragment directed against a cell-surface glycoprotein expressed by most lung cancers, both small cell (SCLC) and non–small cell (NSCLC). OncoScint and Verluma have been largely replaced by PET and are no longer commercially available.
Capromab pendetide (ProstaScint) is an FDA-approved 111In-labeled murine IgG antibody that reacts with a cytoplasmic antigen found in both benign and malignant prostatic cells but not with prostate-specific antigen (PSA) or prostatic acid phosphatase. Concurrent 99mTc-RBC imaging is often utilized to differentiate retained vascular activity from pathologic nodal activity. Fused SPECT/CT images are more useful. Although the images are difficult to interpret, preliminary results indicate that their sensitivity for the detection of pelvic nodal metastases preoperatively and in postprostatectomy patients is much higher than those of CT. ProstaScint scintigraphy is still utilized because of the limitations of 18F-FDG PET for the evaluation of prostate cancer.
Two monoclonal antibodies have also been approved by the FDA for radioimmunotherapy of low-grade or transformed low-grade lymphoma: (a)131I-tositumomab (Bexxar) and (b) yttrium-90 (90Y)-ibritumomab tiuxetan (Zevalin). Discussion related to these agents is beyond the scope of this book.
Whole antibodies are larger molecules that require delayed imaging at 72 to 96 hours because of the slow clearance of background activity; they are thus labeled with 111In. Liver, spleen, and marrow activity are considerable, to the extent that hepatic metastases appear photopenic and are extremely difficult to detect. Antibody fragments, on the other hand, are smaller molecules that can be imaged at 4 and 12 hours owing to rapid renal clearance; therefore they are usually labeled with 99mTc. Significant interfering renal and GI tract activity is often present, although hepatic and marrow activity is less intense.
Because the murine fragments are less antigenic than the whole antibodies, they less frequently result in the formation of human antimouse antibody (HAMA). This immune response is detectable to a variable degree within the first 2 to 3 months following exposure. It is dose-related but may resolve over the ensuing months. The presence of HAMA may result in interference with radioimmunoassays (serum tumor markers, for instance) and alteration of subsequently administered monoclonal antibody biodistribution. Although adverse reactions such as fever, chills, rash, flushing, and angioedema occur in 3% to 4% of patients, they do not increase in frequency or severity in the presence of HAMA. Anaphylactic reactions are rare, but patients are advised to remain in the department for 1 hour after the infusion.
The clinical promise and projected benefits of radioimmunoscintigraphy have yet to be realized, but further developments in molecular biology and immunology are expected to advance this technology.
Integrated PET/CT and SPECT/CT Imaging
A summary of literature regarding the performance of 18F-FDG PET in various malignancies was published in 2001 (8). Although numerous studies have shown that the sensitivity and specificity of 18F-FDG imaging is superior to that of CT in many clinical settings, the inability of 18F-FDG imaging to provide anatomic localization remains a significant impairment in maximizing its clinical utility. Because 18F-FDG is not limited to malignant tissue, the interpreter must be familiar with the normal pattern and physiologic variations of 18F-FDG distribution and with pertinent clinical data to avoid misinterpretations. Limitations of anatomic imaging with CT are well known and related to size criteria to differentiate benign from malignant lymph nodes, difficulty differentiating posttherapy changes from tumor recurrence, and difficulty differentiating nonopacified loops of bowel from metastases in the abdomen and pelvis.
Close correlation of 18F-FDG studies with conventional CT scans helps to minimize these difficulties. Interpretation has traditionally been accomplished by visually comparing corresponding 18F-FDG and CT images. The interpreting physician visually integrates the two image sets in order to precisely locate a region of increased uptake on the CT scan. To aid in image interpretation, computer software has been developed to coregister the 18F-FDG PET emission images with the high-resolution anatomic images provided by CT. An alternative approach that has gained wider acceptance is the hardware approach to image fusion using multimodality imaging with integrated PET/CT and SPECT/CT imaging systems. Design innovations continue to be developed.
Integrated PET/CT and SPECT/CT Systems
The fusion of anatomic and molecular images (PET or SPECT and CT) obtained with integrated PET/CT or SPECT/CT systems, sequentially in time but without moving the patient from the imaging table, allows optimal coregistration of anatomic and molecular images leading to accurate attenuation correction and precise anatomic localization of lesions with increased metabolism. The fusion images provided by these systems allow the most accurate interpretation of both PET/CT and SPECT/CT studies in oncology. Fusion 18F-FDG PET/CT imaging is also a promising tool for optimizing radiation therapy.
Published data regarding the incremental value of integrated PET/CT or SPECT/CT images compared to PET or SPECT alone, or PET or SPECT correlated with a CT obtained at a different time, conclude the following: (a) improvement of lesion detection on both CT and PET or SPECT images, (b) improvement of the localization of foci of uptake resulting in better differentiation of physiologic from pathologic uptake, and (c) precise localization of the malignant foci—for example, in the skeleton versus soft tissue or liver versus adjacent bowel or node. PET/CT and SPECT/CT fusion images affect the clinical management by (a) guiding further procedures, (b) excluding the need of further procedures, and (c) changing both inter- and intramodality therapy. PET/CT fusion images have the potential to provide important information to guide the biopsy of a mass to active regions of the tumor and provide better maps than CT alone to modulate field and dose of radiation therapy (9).
Technical issues regarding optimal protocols and technical and clinical expertise regarding performance and interpretation of PET/CT and SPECT/CT imaging have been discussed (10). Procedure guidelines for tumor imaging using 18F-FDG PET/CT and for SPECT/CT imaging have also been published and list numerous sources of false-positive and false-negative findings (3,11). This new, powerful technology provides more accurate interpretation of both CT and PET or SPECT images and therefore more optimal patient care.
Case 8.1
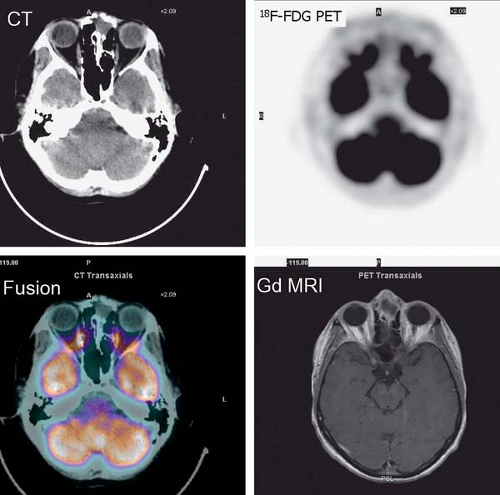
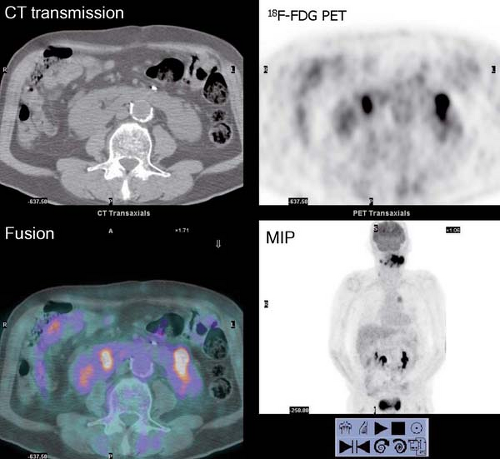
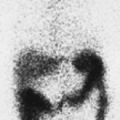
History: A 44-year-old female with a poorly differentiated carcinoma involving the left nasal cavity was treated with chemotherapy. A posttreatment gadolinium (Gd)-enhanced MRI demonstrated a poorly defined soft tissue density in the anterior ethmoid sinuses with peripheral enhancement (Fig. 8.1 A). 18F-FDG PET/CT imaging was performed to differentiate residual viable tumor from posttreatment scarring (Fig. 8.1 B).
Findings: The 18F-FDG PET/CT scan demonstrates no 18F-FDG-avidity in the uptake within the ethmoid sinuses (Fig. 8.1 A). However, mildly increased activity is identified in the endometrial cavity (Fig. 8.1 B).
Discussion: The absence of 18F-FDG activity in the lesion seen on MRI is indicative of a good response to therapy and associated with a good outcome.
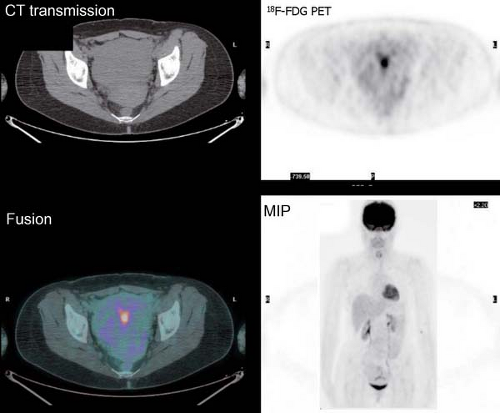
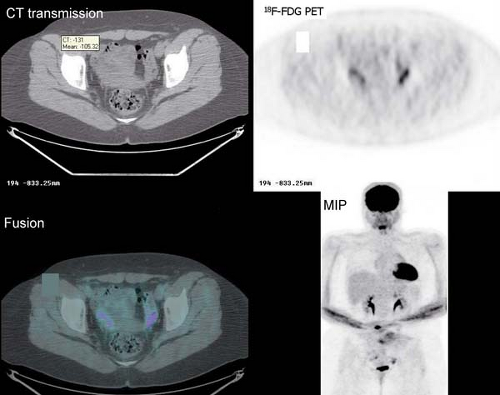
It is essential to be fully familiar with the normal patterns and variations of 18F-FDG biodistribution as well as the numerous benign conditions that accumulate 18F-FDG and can mimic neoplasm. 18F-FDG uptake in the endometrial cavity can be variable, and it may occur especially in menstruating women. During normal menstruation, approximately 40 mL of blood and an additional 35 mL of serous fluid are excreted
into the uterine cavity. This, associated with the inflammatory process due to the necrotic endometrial epithelium from the sudden reduction of estrogen and progesterone levels at the end of the secretory phase of the menstrual cycle, can result in physiologic uterine accumulation. Focal activity in the pelvis can be misinterpreted as a malignant lesion; but the mild degree of uptake and the correct identification of the uterus on the CT transmission images, accompanied by pertinent history, leads to a correct impression of physiologic uptake related to menstruation.
into the uterine cavity. This, associated with the inflammatory process due to the necrotic endometrial epithelium from the sudden reduction of estrogen and progesterone levels at the end of the secretory phase of the menstrual cycle, can result in physiologic uterine accumulation. Focal activity in the pelvis can be misinterpreted as a malignant lesion; but the mild degree of uptake and the correct identification of the uterus on the CT transmission images, accompanied by pertinent history, leads to a correct impression of physiologic uptake related to menstruation.
Physiologic uptake in the ovaries has also been reported in premenopausal women, as illustrated in Figure 8.1 C. In postmenopausal females, however, 18F-FDG uptake in the reproductive organs is associated with a higher incidence of malignancies and warrants further workup (12).
Diagnosis: (a) No abnormal uptake of 18F-FDG in the neck indicative of a good response to therapy. (b) Physiologic uptake in the endometrial cavity due to menstruation.
Case 8.2
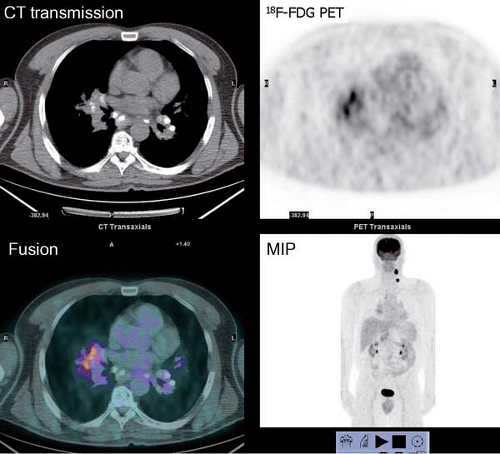
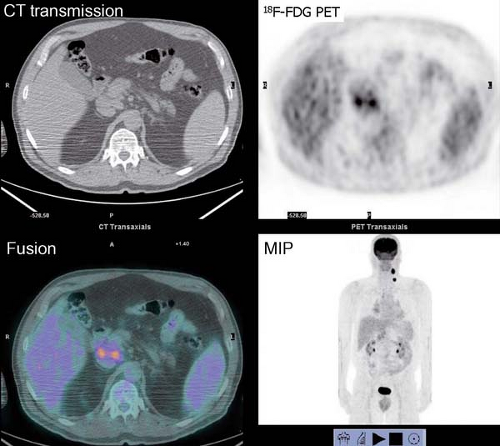
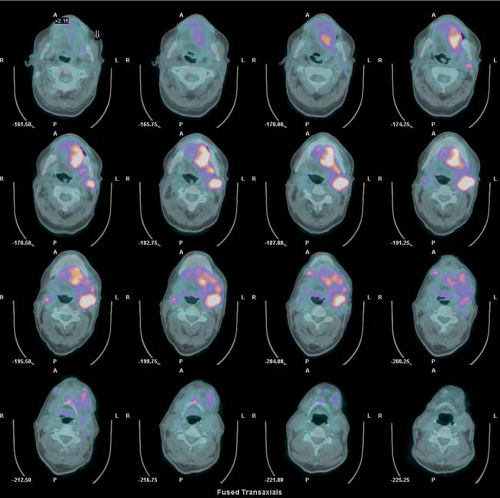
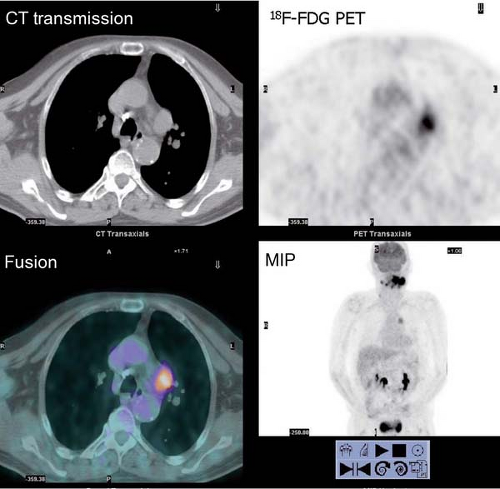
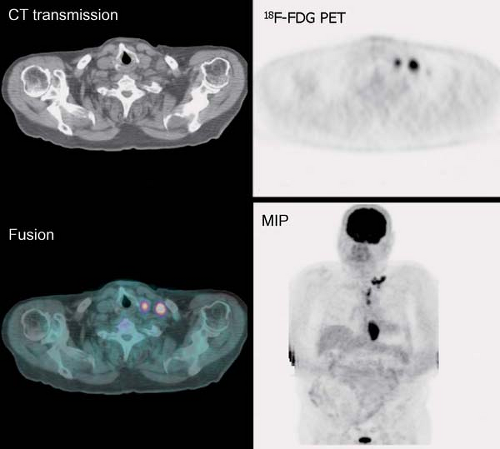
History: One year after undergoing resection of a melanoma of the right ear, a 54-year-old male presented with a palpable lymph node in the left neck. 18F-FDG PET/CT imaging (Figs. 8.2 A and 8.2 B) was performed for restaging.
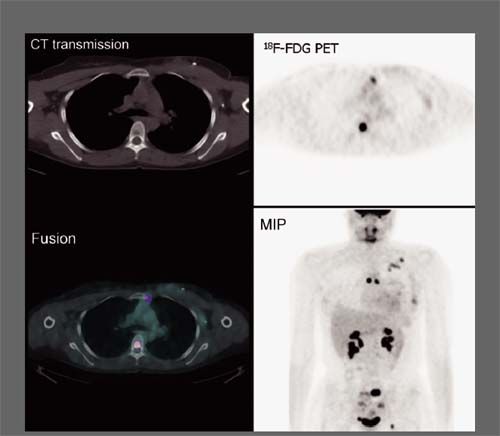
Findings: Two foci of markedly increased uptake are seen in the left neck (Figs. 8.2 A, and 8.2 B), a maximum intensity projection (MIP) image, corresponding on the CT to a 2-cm submandibular and a 2-cm level III lymph node (CT not shown). In addition, there is moderate 18F-FDG uptake in the mediastinum and porta hepatis corresponding to conglomerates of mediastinal, bilateral hilar (Fig. 8.2 A), and porta hepatis (Fig. 8.2 B) lymphadenopathy. The mediastinal nodes are partially calcified on CT.
Discussion: The intense uptake in the two lymph nodes in the neck is consistent with melanoma metastases. The role of PET/CT in evaluation of patients with melanoma is further discussed with Cases 8.19 and 8.20. However, the pattern of moderate uptake in the mediastinum and porta hepatis is not typical for the malignancy of this patient, being more consistent with the patient’s history of sarcoidosis.
Sarcoidosis is a multisystem disorder of unknown etiology most commonly affecting young adults and typically presenting with hilar lymphadenopathy, pulmonary infiltrates, and ocular and cutaneous lesions. The diagnosis is established when typical clinicoradiographic findings are supported by histologic evidence of noncaseating granuloma formation in more than one system unaccompanied by evidence of infection. The sensitivity and specificity of 18F-FDG PET for the detection of malignancy are both greater than 90%, but the false-positive findings have generally been of inflammatory origin,
including granulomatous (tuberculosis, atypical myobacteria, fungus, sarcoidosis, and suture granulomas) and nongranulomatous (bacterial abscess, postradiation, etc.) processes. 18F-FDG PET imaging has also been demonstrated to be highly sensitive for the detection of inflammatory processes related to the high density of activated macrophages associated with inflammation (13,14). There is preliminary evidence that 18F-FDG PET may be useful in monitoring the response of sarcoidosis to therapy and therefore guiding therapeutic intervention.
including granulomatous (tuberculosis, atypical myobacteria, fungus, sarcoidosis, and suture granulomas) and nongranulomatous (bacterial abscess, postradiation, etc.) processes. 18F-FDG PET imaging has also been demonstrated to be highly sensitive for the detection of inflammatory processes related to the high density of activated macrophages associated with inflammation (13,14). There is preliminary evidence that 18F-FDG PET may be useful in monitoring the response of sarcoidosis to therapy and therefore guiding therapeutic intervention.
Diagnosis: (a) Metastatic melanoma restricted to the left neck. (b) Sarcoidosis.
Case 8.3
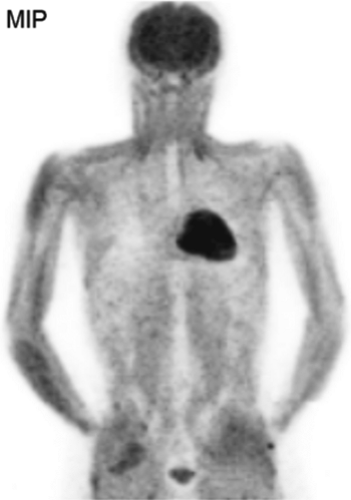
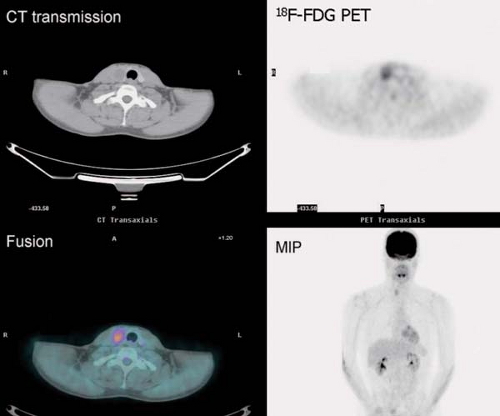
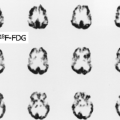
History: A 10-year-old male with previous history of Hodgkin’s lymphoma, stage 3B diagnosed 11 months earlier was referred for 18F-FDG PET/CT imaging 4 months following completion of chemotherapy and radiation therapy. Prior to the 18F-FDG injection, the patient’s serum glucose was 96 mg/dL. A MIP image is displayed in Figure 8.3.
Findings: Intense diffuse symmetrical muscular uptake is seen. No abnormal focal 18F-FDG metabolism is observed. Further history revealed that the patient had not complied with fasting instructions.
Discussion: The key to successful PET imaging is to prepare the patient properly so as to minimize the appearance of artifactual uptake patterns. All patients are instructed to fast for at least 6 hours and preferably 12 hours and to drink plenty of acaloric clear fluids. The serum glucose level should be measured just before 18F-FDG administration to document euglycemia. Postprandial insulin secretion promotes 18F-FDG uptake by skeletal muscle and myocardium, making 18F-FDG less available for tumoral uptake (15). Therefore the sensitivity for detection of metastatic disease is decreased. The same considerations apply to diabetic patients after insulin administration, so administration of 18F-FDG should be delayed for 2 or 3 hours after insulin injection.
Another consideration is 18F-FDG uptake in activated musculature. At rest, skeletal muscle does not show significant accumulation of 18F-FDG, but after exercise or if contraction takes place during the uptake period after the injection of the radiopharmaceutical, there can be marked uptake. Muscular uptake can usually be distinguished from malignant disease because it is often symmetrical and corresponds to the anatomy of muscular groups. Symmetrical uptake in the neck and thoracic paravertebral regions can be produced by patient anxiety alone. Occasionally, uptake can be focal and asymmetrical and very difficult to differentiate from malignant lesions. Therefore patients should be instructed to refrain from strenuous muscular activity for 24 hours prior to 18F-FDG PET imaging. Abstention from talking and chewing during the distribution phase is of prime importance in patients evaluated for head and neck cancer.
Oral benzodiazepine administration should be considered before 18F-FDG injection in patients considered at risk of showing muscle tension or brown fat uptake. Brown fat uptake is discussed in Case 8.25. Diazepam has anxiolytic and muscle-relaxant effects and may offer a simple solution to the diagnostic confusion that can arise between enhanced physiologic muscle uptake and malignant uptake.
Hyperglycemia represents a limitation for the sensitivity of 18F-FDG PET imaging, because the excess of plasma glucose competes with 18F-FDG and consequently reduces 18F-FDG uptake in tumors by up to 50% (16). Therefore control of glycemia in diabetic patients and overnight fasting in normal patients are essential for high-quality 18F-FDG images. The blood glucose level should always be measured before 18F-FDG administration. Not uncommonly, patients with diabetes present with hyperglycemia because they have been instructed to fast overnight and have not appropriately adjusted their medications. Furthermore, unrecognized diabetes is not uncommon in elderly patients with malignancies.
Diagnosis: No evidence of gross recurrent disease; however, the sensitivity of this study for detection of malignant neoplasm is compromised because of diffuse skeletal muscle uptake.
Case 8.4
History: 18F-FDG PET/CT imaging was performed for initial staging of head and neck cancer (Figs. 8.4 A, 8.4 B, 8.4 C and 8.4 D) in a 76-year-old male recently found to have squamous cell carcinoma of the oral cavity extending to the left tonsillar region and submandibular region.
Findings: The fusion 18F-FDG PET/CT images (Fig. 8.4 A) clearly show increased uptake in the floor of the mouth just to the left of midline extending posteriorly into the left parapharyngeal space, corresponding with a large soft tissue density mass on CT transmission images (CT not shown). There is also intense 18F-FDG uptake corresponding to extensive lymphadenopathy in the left and right cervical region (levels I and II) on CT scan and to a right level III node (Fig. 8.4 B). There is also a focus of uptake in the mediastinum corresponding to a 2.3- by 3.2-cm lymph node in the aortopulmonary window (Fig. 8.4 C).
There is an incidental finding of horseshoe kidney (Fig. 8.4 D) and extensive vascular calcification.
Discussion: The findings are consistent with a large primary oropharyngeal tumor with extensive lymph node involvement and probable metastatic disease in the mediastinum, subse- quently confirmed by mediastinoscopy and lymph node biopsy (stage IVC). He was treated with chemo- and radiation therapy.
For accurate staging of head and neck cancer, it is very important to be familiar with the TNM staging system recently updated by the American Joint Committee on Cancer (AJCC) (17). Although each primary site of tumor has a site-specific staging classification, typically primary tumors are staged according to size, from T1 to T4, with T4 indicating invasion of adjacent vessels, muscle, bone, or cartilage. For cervical nodes, N1 denotes a single ipsilateral node less than 3 cm, N2 indicates single or multiple nodes between 3 and 6 cm, and N3 refers to node(s) greater than 6 cm in greatest dimension. For all head and neck cancers regardless of the primary site, involvement
of neck nodes has important therapeutic and prognostic implications. Therefore the knowledge of nodal anatomy and the standard system for describing the location of nodes is critical for proper communication with referring surgeons (18).
of neck nodes has important therapeutic and prognostic implications. Therefore the knowledge of nodal anatomy and the standard system for describing the location of nodes is critical for proper communication with referring surgeons (18).
For initial staging of head and neck carcinoma, 18F-FDG PET has a 10% advantage in sensitivity and specificity over CT, MRI, or ultrasound (US) (19,20). The reported change in management due to PET is in the range of 8% to 15% of the cases. As the accuracy of 18F-FDG PET is superior to CT and MRI, 18F-FDG PET/CT is recommended in the preoperative staging of patients with head and neck cancer. The high specificity (about 90%) and positive predictive value (PPV) (about 85%) of a positive PET scan indicates the need for neck dissection. The negative predictive value (NPV) is in the 90% range on a patient basis and there is still a debate regarding neck dissection in the N0 neck patient, since microscopic disease is below the resolution of any imaging modality and does occur in some 10% to 20% of patients (21,22).
In the post-therapy setting, there is a consensus that PET/CT has a clear advantage over CT or MR imaging in the evaluation of head and neck carcinoma. However, interpretation of 18F-FDG PET images should be performed with the surgical and treatment history and correlation with CT and MRI for precise localization of the lesions (23,24).
18F-FDG PET is also helpful for detection of the primary lesion of metastatic cancer of unknown primary (CUP). 18F-FDG PET detects the primary in 43% (range 8% to 65%) of patients, which approximately doubles the rate of detection compared with conventional diagnostic procedures (25).
As for other primaries, the performance of PET/CT has been compared with that of PET alone. PET/CT aids in precisely localizing lesions and decreasing the number of equivocal lesions by 53%, resulting in a higher accuracy than PET alone (96% versus 90%) and a favorable impact on management in 18% of patients (26).
18F-FDG is excreted by the kidneys, and transaxial and coronal images are usually most helpful in differentiating renal uptake from uptake in another anatomic structure. In this patient, the pattern of uptake clearly shows the shape of a horseshoe kidney. Unrecognized renal transplants can appear as malignant lesions. Although the absence of the native kidneys should be a warning sign, simultaneously acquired CT images delineate the anatomy and help avoid false-positive findings. Focal accumulation in the ureters is a common finding due to the pooling of radiotracer in the recumbent patient, although the intensity and location of uptake usually allow accurate identification of the ureters in patients with abdominal malignancies, especially on the coronal projections.
Diagnosis: (a) Carcinoma in the oral cavity. (b) Extensive bilateral metastatic cervical lymphadenopathy. (c) Metastatic focus in the aortopulmonary window, elevating the patient’s disease to stage IV and changing management. (d) Horseshoe kidney.
Case 8.5
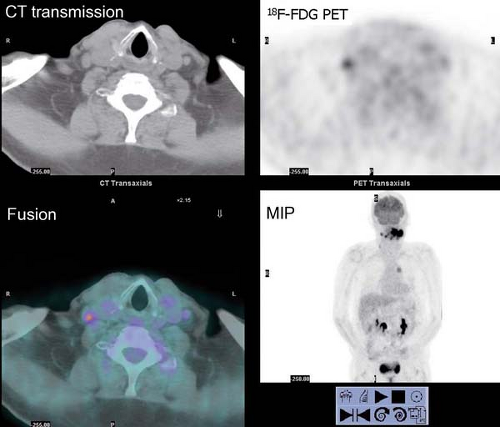
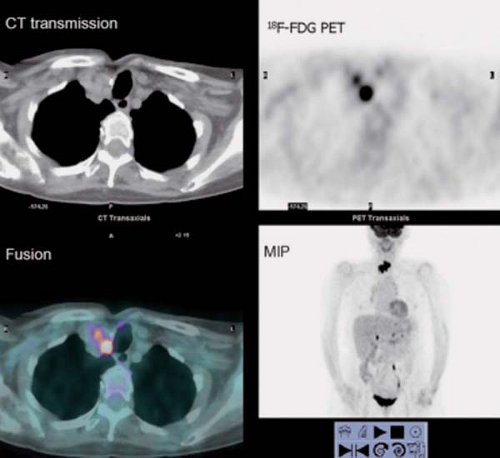
History: This 43-year-old man was recently diagnosed with medullary carcinoma of the thyroid (MCT) on the basis of fine-needle aspiration (FNA) biopsy. The patient had not undergone any therapy. He was referred for 18F-FDG PET/CT imaging (Fig. 8.5) for initial staging.
Findings: PET/CT imaging reveals a 2.1- by 2.0-cm solid nodule within the right lobe of the thyroid, which is moderately 18F-FDG–avid, as is usually seen in MCT.
Discussion: 18F-FDG PET is currently the most accurate technique for the detection of persistent/recurrent MCT and is more sensitive than CT or MRI (27). All imaging modalities are dependent on volume of disease and size of lesions for the detection of metastatic and recurrent MCT. While the overall sensitivity for detection of disease with 18F-FDG PET/CT is only 63%, the sensitivity rises to 78% when the serum calcitonin is >1,000 pg/mL. With low-volume disease, sensitivity is quite low, 20% with serum calcitonin <1,000 pg/mL and 0% with calcitonin <500 pg/mL. When 18F-FDG PET/CT is negative, conventional imaging and SSR imaging is also often negative (28).
Medullary carcinoma of the thyroid, representing approximately 3% to 5% of all thyroid cancers, is an intermediate-grade malignancy occurring in both sporadic (80%) and familial (20%) forms. There is an autosomal dominant inheritance in families with multiple endocrine neoplasia (MEN) 2A and 2B. Nearly 50% of patients have metastatic cervical adenopathy at presentation. With clinically palpable disease, surgical cure is rarely achieved, despite regional lymphadenectomy, but the 10-year survival rate is 86% even with persistent postoperative
hypercalcitoninemia (29). Five-year survival is 94% in patients with metastatic lymphadenopathy but only 41% in those with extranodal disease (30). Only in the occasional patient with MCT is the tumor radioiodine-avid, so 131I scintigraphy is not useful and 131I treatment results in no improvement in survival or recurrence rate (31).
hypercalcitoninemia (29). Five-year survival is 94% in patients with metastatic lymphadenopathy but only 41% in those with extranodal disease (30). Only in the occasional patient with MCT is the tumor radioiodine-avid, so 131I scintigraphy is not useful and 131I treatment results in no improvement in survival or recurrence rate (31).
Surgery is currently the only potentially curative approach to the management of MCT. Persistent or recurrent disease is easily detected by elevated serum levels of tumor markers, calcitonin and carcinoembryonic antigen (CEA), but localization of persistent/recurrent disease remains a diagnostic problem. Most medullary tumors express SSRs, and the majority of patients with slow-growing or occult disease will have a positive localization with 111In-octreotide scintigraphy, although the extent of disease may be underestimated (32). Like other neuroectodermal tumors, 40% to 50% of MCTs will take up 123I/131I-MIBG (33). Although less sensitive than 201Tl, 99mTc-(V)-DMSA, and 99mTc-MIBI, 131I-MIBG is poorly sensitive (about 30%) but highly specific and may thus be useful in conjunction with other localization modalities. Furthermore, the therapeutic efficacy of high-dose (100 to 300 mCi) 131I-MIBG in patients with significant uptake appears promising (34,35). Although a complete response is uncommon, a partial response with significant symptom palliation and hormonal improvement has occurred in the majority of the cases.
In summary, 18F-FDG PET appears to be the modality of choice for evaluation of patients with suspicion of recurrent MCT, especially in those with low-volume disease. There are few data regarding the role of scintigraphic imaging at initial staging.
Diagnosis: Primary medullary thyroid carcinoma without evidence of metastases.
Case 8.6
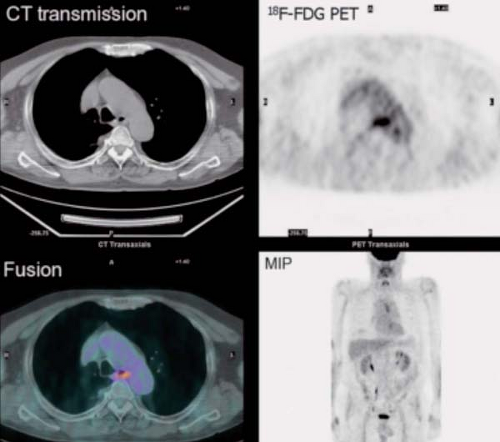
History: A 78-year-old male with a history of papillary thyroid carcinoma treated by total thyroidectomy presented with a serum thyroglobulin elevated to 11.9 ng/mL. On three occasions, he had been treated with high-dose 131I therapy. The most recent post-therapy 131I whole-body scan revealed no evidence of cervical or distant radioiodine-avid metastases. 18F-FDG PET/CT was requested for further assessment (Fig. 8.6). The patient received parenteral recombinant TSH 24 hours and 48 hours prior to administration of 18F-FDG.
Findings: 18F-FDG PET/CT images reveal a large conglomerate of intensely increased activity at the base of the neck in the midline extending into the right neck. The most inferior foci in the right neck shown on the transaxial images correspond to subcentimeter lymph nodes.
Discussion: These findings are consistent with metastatic thyroid carcinoma. A conglomerate of metastatic nodes was subsequently resected from the neck; 4 months postoperatively, serum thyroglobulin was undetectable and a repeat CT of the neck was unrevealing, indicative of a complete response to therapy.
Whole-body 131I scintigraphy has a sensitivity of 60% to 70% for the detection of metastases, in part dependent upon the dose of radioiodine utilized. Metastases are more often
seen with a 10-mCi diagnostic dose of 131I than with a 2- to 5-mCi dose, and posttherapy scans obtained 5 to 8 days after a >100-mCi dose of 131I reveal additional lesions or clarify equivocal abnormalities seen on a diagnostic scan in as many as 35% of patients. The visualization of 131I-avid metastases is of utmost importance in view of the well-demonstrated efficacy of 131I therapy in patients with such disease. However, as these differentiated neoplasms are subjected to various therapies, they may dedifferentiate, losing the iodine symporter and thus failing to accumulate radioiodine while retaining the ability to express thyroglobulin. Other differentiated thyroid neoplasms as well as less differentiated ones—including Hurthle cell, medullary, and anaplastic carcinomas—are not 131I-avid. These thyroid neoplasms not detected by 131I scintigraphy have been successfully imaged using 201Tl, 99mTc-sestamibi, and 99mTc-tetrofosmin, but 18F-FDG PET has demonstrated high sensitivity and specificity for the detection of these tumors and their metastases.
seen with a 10-mCi diagnostic dose of 131I than with a 2- to 5-mCi dose, and posttherapy scans obtained 5 to 8 days after a >100-mCi dose of 131I reveal additional lesions or clarify equivocal abnormalities seen on a diagnostic scan in as many as 35% of patients. The visualization of 131I-avid metastases is of utmost importance in view of the well-demonstrated efficacy of 131I therapy in patients with such disease. However, as these differentiated neoplasms are subjected to various therapies, they may dedifferentiate, losing the iodine symporter and thus failing to accumulate radioiodine while retaining the ability to express thyroglobulin. Other differentiated thyroid neoplasms as well as less differentiated ones—including Hurthle cell, medullary, and anaplastic carcinomas—are not 131I-avid. These thyroid neoplasms not detected by 131I scintigraphy have been successfully imaged using 201Tl, 99mTc-sestamibi, and 99mTc-tetrofosmin, but 18F-FDG PET has demonstrated high sensitivity and specificity for the detection of these tumors and their metastases.
Although 18F-FDG PET may demonstrate some 131I-avid tumors, a heterogeneous population of 131I-positive/18F-FDG-negative and 131I-negative/18F-FDG-positive metastases may coexist in an individual patient. In an unselected population of patients with thyroid cancer, the sensitivity of 18F-FDG PET is in the range of 50% to 75%. 18F-FDG PET has proved most useful in the population of thyroid carcinoma patients who are suspected of harboring metastases due to elevated serum thyroglobulin but in whom conventional imaging and 131I whole-body scintigraphy are negative, with a sensitivity in the range of 70% to 90%. PET has localized occult metastases in 50% to 82% of such patients and altered therapy in about 30% (36). High 18F-FDG uptake indicates a poor prognosis, especially if there is high-volume disease (37). TSH stimulation enhances 18F-FDG uptake by malignant thyroid tumors; therefore rhTSH or thyroid hormone withdrawal is recommended to improve the sensitivity for detection of recurrent and/or metastatic disease (38).
In summary, 131I scintigraphy is the preferred modality for the detection of recurrent/metastatic differentiated thyroid carcinoma because of its ability to identify a tumor treatable with high-dose radioiodine administration. In patients suspected of metastatic disease but in whom 131I imaging is unrevealing, 18F-FDG PET offers high sensitivity and specificity for the detection of metastases, which may then be treated by surgical resection or external radiotherapy. TSH stimulation by thyroid hormone withdrawal or rhTSH administration enhances sensitivity. 18F-FDG imaging is also helpful in patients with Hurthle cell carcinoma (39), medullary carcinoma, and probably anaplastic carcinoma.
Diagnosis: Radioiodine-negative, 18F-FDG-positive meta- static thyroid cancer.
Case 8.7
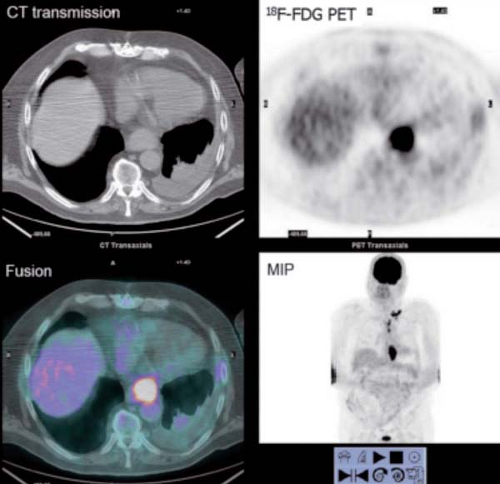
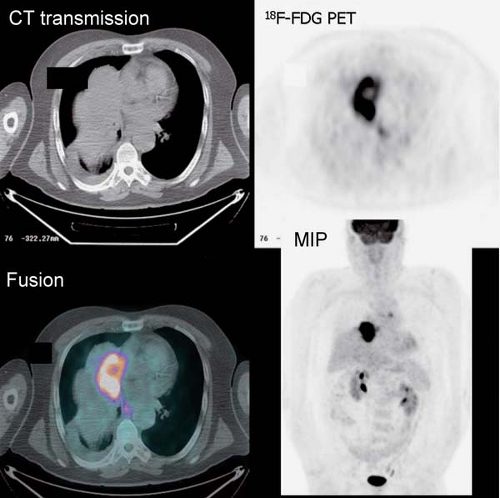
History: An 80-year-old male was referred for initial staging of esophageal carcinoma using 18F-FDG PET/CT imaging (Figs. 8.7 A and 8.7 B).
Findings: The images reveal marked 18F-FDG uptake along the distal esophagus, corresponding to the portion of the esophagus with marked thickening on the CT scan (Fig. 8.7 B) and consistent with the patient’s known primary esophageal carcinoma. In addition, multiple foci of 18F-FDG uptake are seen in the mediastinum (paraesophageal and paratracheal) and left supraclavicular regions, corresponding to lymph nodes seen on the CT images (Fig. 8.7 B) and indicative of metastases.
Discussion: These findings are consistent with the patient’s known primary esophageal carcinoma with involvement of mediastinal and left supraclavicular lymph nodes. This case demonstrates the value of preoperative 18F-FDG PET imaging in the evaluation of esophageal carcinoma. 18F-FDG PET is more sensitive than CT at detecting distant metastasis and at least as sensitive as CT in detecting regional metastasis. This is of critical importance, since approximately 20% of patients with esophageal cancer being considered for surgical therapy have distant metastases (40). 18F-FDG PET also demonstrates the primary tumor in the vast majority of patients. Both CT and PET are limited by their inability to determine the depth of wall invasion; however, endoscopic ultrasound (EUS) is useful in this regard. When 18F-FDG PET is performed in addition to CT, ill-advised surgery may be decreased by 90%. PET may play a role in differentiating responders from nonresponders after chemotherapy as well. Therefore PET imaging followed by EUS with FNA biopsy of abnormal lymph nodes or
metastases provides the most cost-effective strategy for preoperative staging and management of patients with carcinoma of the esophagus (41).
metastases provides the most cost-effective strategy for preoperative staging and management of patients with carcinoma of the esophagus (41).
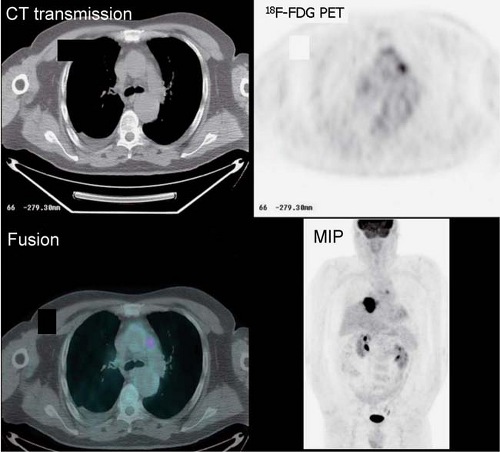
Esophageal carcinoma is often treated preoperatively with neoadjuvant chemoradiation therapy, and 18F-FDG PET is useful to assess the response to therapy prior to surgery. After radiation therapy, radiation-induced esophagitis occurs; these inflammatory changes should not be confused with residual tumor Fig. 8.6 C, as illustrated from a different patient with lung cancer who underwent PET/CT imaging 2 weeks following chemoradiation therapy. The uptake matches the radiation field and corresponds to a normal esophagus on the CT scan.
Diagnosis: Distal esophageal carcinoma with evidence of mediastinal and left supraclavicular metastases.
Case 8.8
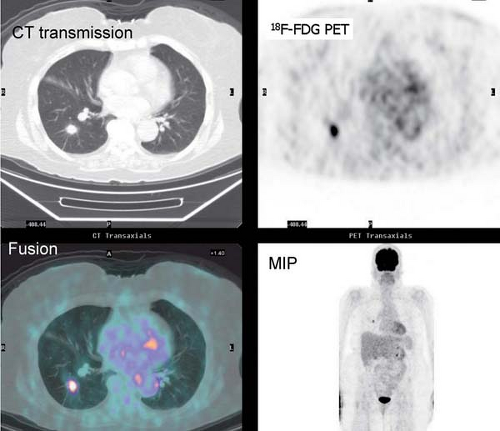
History: This 76-year-old female presented with a 1.5-cm pulmonary nodule in the right lower lobe. 18F-FDG PET/CT imaging was performed to further characterize the lesion (Fig. 8.8)
Findings: The pulmonary nodule in the right lower lobe demonstrates moderate 18F-FDG avidity above that of normal liver parenchyma. No evidence of hypermetabolic foci is noted in the mediastinum.
Discussion: These findings indicate that the pulmonary nodule is likely malignant; a less likely explanation includes granulomatous infection. A biopsy demonstrated carcinoma.
In the United States, 130,000 new solitary pulmonary nodules (SPNs) are diagnosed annually, but only 30% to 50% are malignant. Chest radiography and CT have well-known limitations for differentiating benign from malignant nodules. This leads to a large number of invasive procedures. Transbronchial and percutaneous lung biopsy suffer from sampling error. In addition, percutaneous needle biopsy may be complicated by a pneumothorax, requiring chest tube placement in up to 5% to 10% of these patients (42). Functional imaging with 18F-FDG can identify malignant lesions with a sensitivity ranging from 77% to 100% and an accuracy of 81% to 94%. In a meta-analysis including 40 studies and 1,474 focal pulmonary lesions, the maximum joint sensitivity and specificity of ROC curve was 91%; the authors concluded that to decrease the false-negative rate, 18F-FDG PET must operate at sensitivity of 96% and specificity 78% (43). It has been suggested that some of these variations may be related to the regional prevalence
of granulomatous diseases. False-negative exams are generally due to small-sized lesions relative to scanner resolution (partial volume effect), low cellularity of lesions (mucinous tumors) (44), or low metabolic activity of tumors—bronchioalveolar carcinoma (45) and carcinoid tumors (46). Lesions that are smaller than twice the size of the resolution of the imaging system at full width at half maximum suffer from partial volume averaging and may be falsely negative. The resolution of the newest state-of-the-art dedicated PET camera is 4 to 5 mm; therefore lesions greater than 1 cm in size can be evaluated without concern for partial volume averaging artifact.
of granulomatous diseases. False-negative exams are generally due to small-sized lesions relative to scanner resolution (partial volume effect), low cellularity of lesions (mucinous tumors) (44), or low metabolic activity of tumors—bronchioalveolar carcinoma (45) and carcinoid tumors (46). Lesions that are smaller than twice the size of the resolution of the imaging system at full width at half maximum suffer from partial volume averaging and may be falsely negative. The resolution of the newest state-of-the-art dedicated PET camera is 4 to 5 mm; therefore lesions greater than 1 cm in size can be evaluated without concern for partial volume averaging artifact.
Semiquantitative indices have been investigated to determine an optimal threshold of uptake to separate benign from malignant lesions. The most popular indices are the standardized uptake value (SUV) and the lesion to lung background ratio (L/B). However, SUV measurement requires rigorous quality control for accuracy as described in the introduction. Visual interpretation of the degree of uptake relative to normal liver parenchyma (average SUV about 2.0) is often adequate for clinical purposes. An SUV of 2.5 is accepted by most centers as the best threshold to differentiate benign from malignant lesions in the lungs. If a lesion greater than 1 cm in diameter has an SUV less than 2.5, malignancy can be excluded with a high degree of certainty. However, patients should always be managed by clinical and periodic imaging follow-up for 24 months. On the other hand, a positive study is not specific and requires further testing.
An analysis of potential cost benefits projects that appropriate utilization of 18F-FDG PET in the evaluation of SPNs would save approximately 180 million in annual health-care costs in the United States by decreasing the number of thoracotomies and percutaneous needle biopsies performed (47).
Diagnosis: Malignant solitary pulmonary nodule.
Case 8.9
History: A biopsy of a right hilar mass in a 66-year-old male revealed non–small cell carcinoma (NSCLC). 18F-FDG PET/CT was performed for initial staging (Figs. 8.9 A and 8.9 B).
Findings: There is increased 18F-FDG uptake in the large right hilar mass with central photopenia, but the mass is much larger than the extent of uptake. There is also uptake in a subcentimeter paraesophageal node and a subcentimeter aortopulmonary lymph node. In addition there is a small right pleural effusion with partial consolidation. There is an aneurysm of the ascending and descending aorta.
Discussion: The image findings represent a stage IIIB NSCLC which is not curable by surgery. The landmark of stage IIIB is the presence of metastatic contralateral lymph nodes (N3). In this case, the lymph nodes do not meet size criteria for malignant involvement by CT, so PET was critical for optimal management. In addition the tumor is surrounded by a large area of atelectasism which has implications for determining the field of radiation therapy, as discussed in the introduction to this chapter (9). Using PET/CT, the gross target volume (GTV) is altered (increased or decreased by 25%) in up to 56% of cases. The GTV variability between two independent oncologists is decreased and the treatment is changed from curative to palliative in some 15% of patients.
For accurate staging, it is very important to be familiar with the TNM staging system for NSCLC, recently updated by the AJCC (48). It is also very important to be familiar with the 1996 AJCC–Union Internationale Contre le Cancer (UICC) regional lymph node classification for lung cancer staging (49). Surgical resection is the treatment of choice for patients who have unilateral disease, including ipsilateral lymph nodes (stage N2). Involvement of contralateral lymph nodes (stage N3) is, however, a contraindication to surgery. The criteria for determining lymph node involvement on anatomic imaging studies (CT and MRI) are based on shape and size only. These criteria have obvious limitations, as small lymph nodes may be involved by
tumor and benign inflammatory nodes may be enlarged. In a 1997 report, sensitivity and specificity of CT and MRI for detecting mediastinal involvement was 48% to 52% and 64% to 69% respectively (50). Twenty percent of patients with a negative CT have positive mediastinoscopy (51), and 15% of patients with a negative mediastinoscopy have N2 disease at surgery (52). Therefore CT cannot replace mediastinoscopy for accurate staging of the mediastinum, but mediastinoscopy is limited to the anterior mediastinum in addition to sampling error do underestimate mediastinal involvement.
tumor and benign inflammatory nodes may be enlarged. In a 1997 report, sensitivity and specificity of CT and MRI for detecting mediastinal involvement was 48% to 52% and 64% to 69% respectively (50). Twenty percent of patients with a negative CT have positive mediastinoscopy (51), and 15% of patients with a negative mediastinoscopy have N2 disease at surgery (52). Therefore CT cannot replace mediastinoscopy for accurate staging of the mediastinum, but mediastinoscopy is limited to the anterior mediastinum in addition to sampling error do underestimate mediastinal involvement.
18F-FDG imaging detects mediastinal involvement with a sensitivity of 76% to 89% and specificity of 81% to 99%; however, 18F-FDG PET does not permit evaluation of bronchial, vascular, and pleural involvement and therefore should be performed in addition to (not in place of) CT (53). In a meta-analysis including 14 studies (514 patients) for 18F-FDG PET and 29 studies (2,226 patients) for CT, both the sensitivity and specificity of 18F-FDG PET were superior to CT (79% versus 60% for sensitivity and 91% versus 77% for specificity) for staging the mediastinum (54). In a prospective study of 102 patients, the sensitivity of PET was 91% compared to 75% for CT, the specificity of PET was 86% compared to 77% for CT, and PET detected distant metastases in 10% of patients (55). The addition of PET to the conventional workup is cost-effective (56). PET/CT has proven to be more effective than the simple concomitant analysis of PET and CT alone (57).
If a PET/CT does not demonstrate mediastinal or distant metastases, surgery may proceed without mediastinoscopy. A positive PET showing the primary site and evidence of mediastinal involvement requires mediastinoscopy, not only to exclude false positive findings but also to separate patients with minimal N2 disease (that may be treated with neoadjuvant chemotherapy with subsequent surgery) from patients with more extensive N2 or N3 disease (58).
The effectiveness of PET in the staging of patients with NSCLC has been shown to change patient management in 19% to 41% of cases. Of particular importance is the exclusion of surgery by demonstration of unsuspected distant metastases. The multicenter PLUS trial included 188 patients from 9 hospitals who were randomized to conventional workup (CWU) and CWU plus PET. The endpoint was futile thoracotomy. This trial demonstrated that PET decreased futile thoracotomies in 50% of patients. There were 41% futile thoracotomies with CWU and only 21% with CWU plus PET.
Cerebral, skeletal, and adrenal metastases are common in patients with lung cancer and indicative of incurable stage IV disease. 18F-FDG has limited sensitivity (about 60%) for detection of cerebral metastases because of the high physiologic uptake in the gray matter; MRI remains the standard of care. However, if the brain is in the field of view, careful examination will reveal unexpected metastases in some 0.5% of cases (59). For detection of skeletal metastases, 18F-FDG PET has a sensitivity in the same range as skeletal scintigraphy but is much more specific (60). The topic of detection of skeletal metastases is further discussed in Case 8.11.
Incidental adrenal masses are identified in approximately 2% of patients undergoing CT imaging for reasons other than an adrenal mass. In patients without a known malignancy, the likelihood of an incidental (<5 cm) adrenal mass representing a malignant neoplasm is very low. After an appropriate endocrinologic evaluation to exclude a functioning adrenal neoplasm, a follow-up CT without biopsy is generally recommended. In patients with a known malignancy, an incidental adrenal mass may represent metastasis in 27% to 36% of patients; in such patients, accurate diagnosis is important for treatment planning. Many of these patients undergo percutaneous needle biopsy, which has an accuracy of 80% to 100%. On noncontrasted CT, most metastases are over 20 Hounsfield units (HU), and 85% of adenomas are <18 HU. All masses with <2 HU are benign. Metastases enhance more intensely than do benign lesions. Looking at attenuation with contrast enhancement and delayed contrast washout is also helpful to the extent that >60% enhancement washout at 15 minutes is indicative of benignity.
MRI is also emerging as a useful modality in differentiating benign from malignant adrenal and bone lesions. In a series of 20 patients with 24 incidental adrenal masses and a known malignancy, 18F-FDG PET was able to separate benign (10) from malignant (14) lesions in all cases (61). Benign adrenal adenomas have 18F-FDG activity less than or similar to that of normal liver parenchyma. 18F-FDG PET is the modality of choice for masses indeterminate on CT and is a preferable technique in patients with known malignancy for staging the whole body.
PET has an important role in the evaluation of patients with small cell lung cancer. Patients are staged in a binary distinction between limited disease, with tumor confined to one hemithorax, and with extensive disease. Therefore accurate staging is important, since this determines subsequent therapy and prognosis. Several studies have demonstrated the potential usefulness of 18F-FDG PET as a simplified staging tool in these patients (62). In treated patients, survival is lower in patients with PET-positive findings than in patients with PET-negative results; SUV max has a significant negative correlation with survival (63).
Diagnosis: (a) NSCLC, stage IIIB. (b) Large area of postobstructive atelectasis.
Case 8.10
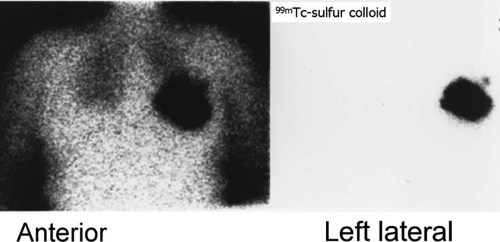
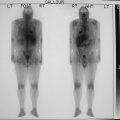
History: A 62-year-old woman was found to have carcinoma upon FNA biopsy of a left breast mass. After the intradermal injection of 240 μCi of filtered 99mTc–sulfur colloid in a circumferential pattern around the breast mass, delayed static images were acquired prior to lumpectomy and excisional biopsy of the axillary sentinel node (Fig. 8.10 A).
Findings: A solitary focus of increased activity is identified in the left axilla on the left lateral view (Fig. 8.10 A). Because of the large amount of activity in the breast, the lesion is more difficult to visualize on the anterior views; the skin was tattooed to aid localization of the sentinel node at surgery performed later that morning.
Discussion: The accumulation of radiolabeled colloid merely identifies the first lymph node to which the breast mass drains; it does not indicate pathology. Following lumpectomy, a handheld gamma probe was used intraoperatively to identify the axillary sentinel node for excisional biopsy.
A good review of this topic is available (64). There is strong evidence that the histopathology of the sentinel node does predict involvement of the other nodes in the region. The sentinel node(s) can be localized at minimally invasive surgery in 92% to 100% of patients using an intraoperative probe. False-negative sentinel nodes have been reported in <3% of patients, but many of these have occurred in patients with multicentric tumors. In fact, one investigator has reported a higher incidence (43%) of tumor when only the sentinel node was examined than when all the nodes within the axilla (29%) were examined, indicative of more accurate staging. The routine use of lymphoscintigraphy in patients with clinically negative axillary nodes results in the avoidance of axillary lymphadenectomy in the patients who do not have lymphatic tumor and reduces morbidity and costs considerably.
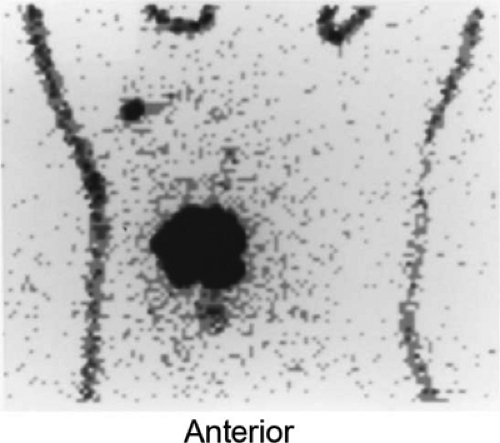
Furthermore, lymphoscintigraphy has documented drain- age occurring across the breast to internal mammary or axill- ary nodes in 32% of patients with outer- or inner-quadrant lesions; drainage of an upper outer-quadrant mass may cross to the internal mammary chain in as many as 56% of patients (65). This is illustrated in another patient with a biopsy-proven breast carcinoma in the inferomedial quadrant of her right breast who underwent lymphoscintigraphy (Fig. 8.10 B). In addition to the right axillary sentinel node easily seen on the outlined body profile, there is faint activity in an internal mammary node superior to the medial border of the breast.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree