This chapter reviews tumor scintigraphy using radiopharmaceuticals other than F-18 fluorodeoxyglucose (FDG), as well as therapeutic radiopharmaceuticals for specific malignancies ( Box 13.1 ). Theranostics is a topic of increasing importance as it relates to nuclear oncology. The term refers to a pharmaceutical that is labeled with one radionuclide for diagnostic imaging and another radionuclide for therapy, for example, Ga-68 dotatate and Lu-177 dotatate for neuroendocrine tumors and Ga-68 PSMA or F-18 prostate-specific membrane antigen (PSMA) and Lu-111 PSMA for prostate cancer. Although this approach is not really new, with radioiodine being the first theranostic agent used for diagnosis and treatment, the field is rapidly growing thanks to the many new agents entering the clinical arena.
Tumor-Type Specific
Iodine-131: Thyroid iodine uptake (papillary and follicular thyroid cancer)
Iodine-131 mIBG: Adrenal medullary uptake in neural crest tumors
Radiolabeled Antibodies, Tumor Antigens
In-111 capromab pendetide (ProstaScint): Monoclonal antibody to intracellular epitope of PSMA (prostate cancer)
F-18 fluciclovine (Axumin): Amino acid upregulated in carcinomas (prostate cancer)
Ga-67-, F-18-, Tc-99m-, and Lu-177-labeled small antibody to extracellular PSMA antigen (prostate cancer)
In-111 ibritumomab tiuxetan (Zevalin): Monoclonal C-20 antibody (lymphoma)
Radiolabeled Peptides: Somatostatin Receptors
In-111 pentetreotide (OctreoScan): Targets somatostatin receptors in neuroendocrine tumors
Ga-68 dotatate (NetSpot): Targets somatostatin receptors in neuroendocrine tumors for diagnosis
Lu-177 dotatate (Lutathera): targets somatostatin receptors in neuroendocrine tumors for therapy
Nonspecific Mechanisms of Tumor Uptake
F-18 fluorodeoxyglucose: Glucose metabolism
Ga-67 citrate: Iron binding
Tc-99m sestamibi: Mitochondrial attraction (breast cancer, renal oncocytomas)
PSMA, Prostate-specific membrane antigen.
Neuroendocrine Tumor Imaging and Peptide Receptor Radiotherapy
Gastroenteropancreatic and Lung Neuroendocrine Tumors
Neuroendocrine tumors (NETs) are a diverse group of epithelial neoplasms that may occur in almost any organ but most commonly arise in the gastroenteropancreatic region (70%) and lung (20%). Well-differentiated neuroendocrine tumors in these regions were previously called carcinoids ( Fig. 13.1 ). Although they often produce specific clinical syndromes due to their unique secretory products (“carcinoid syndrome”), the majority of the tumors are nonfunctioning. Many NET tumors are well differentiated, and these patients have prolonged survival; however, some grow more aggressively and become metastatic. The initial clinical diagnosis can be difficult because of their nonspecific clinical manifestations, depending on the specific amines/peptides secreted, and their small size ( Table 13.1 ). Gastroenteropancreatic NETs are classified on the basis of the Ki-67 proliferation index or the mitotic count ( Table 13.2 ).

| Site | Tumor | Peptide/Amine | Clinical Features |
|---|---|---|---|
| Foregut | Carcinoids: Bronchi, thymus, stomach, first part of duodenum, pancreas | Histamine, ACTH, CRH, GH, gastric, 5 HIAA, 5 HTP | Pulmonary obstruction, flush, hormone syndrome |
| Midgut | Carcinoids: Second part of duodenum, jejunum, ileum, right colon | 5-HT, tachykinins, prostaglandins, bradykinins, 5-HIAA | Bowel obstruction, flush, wheeze, diarrhea |
| Hindgut (distal third of transverse colon and splenic flexure, descending colon, sigmoid colon, and rectum) | Insulinoma Gastrinoma VIPoma Glucagonoma Somatostatinoma GRFoma ACTHoma | Insulin, proinsulin Gastrin VIP Glucagon SS GRF ACTH | Whipple’ triad Zollinger, Ellison Watery diarrhea, hypokalemia DM, cachexia Gallstones, DM, steatorrhea Acromegaly Cushing’ syndrome |
| Histological Classification | Well Differentiated (Low Grade) | Moderately Differentiated (Intermediate Grade) | Poorly Differentiated (High Grade) |
|---|---|---|---|
| Prognosis | Prolonged survival | Intermediate | Poor |
| Mitotic rate | <2 | 2–20 | >20 |
| Ki-67 index | <3% | 3–20% | >20% |
| Necrosis | Absent | Not well defined | Present |
Resection of NETs can potentially cure the patient of the malignancy and amine/peptide production; however, this is often not possible due to the extent of disease. Management guidelines emphasize that resection should be the first-line treatment for patients with advanced tumors if 90% of the disease burden is resectable. However, only 5% to 20% of patients meet this criterion. Liver-directed therapies (embolization, radiofrequency ablation, chemoembolization, and radioembolization) are used in appropriate cases. Chemotherapy has not been very effective, but somatostatin (Sandostatin), mTOR inhibitors (everolimus), and tyrosine kinase inhibitors (sunitinib) can extend survival. With metastatic disease, 5-year survival rates are less than 50%.
Computed tomography (CT), ultrasonography, and magnetic resonance imaging (MRI) are used for initial evaluation, but detection rates are not high due to the small size of the tumors and variable location. Because well-differentiated NETs express high levels of somatostatin receptors (SSTRs), SSTR-targeted radionuclide imaging (e.g., In-111 pentetreotide or Ga-68 dotatate) can detect and functionally characterize these tumors. The primary tumor and regional or distant metastases can also often be detected. Expression of SSTRs is associated with a good prognosis, whereas lack of expression of SSTRs and overexpression of GLUT (FDG uptake) is predictive of poor prognosis and survival.
The normal human hormone somatostatin is a 14-amino-acid peptide produced in the hypothalamus, pituitary gland, brainstem, gastrointestinal tract, and pancreas. Somatostatin receptors are found on many normal cells as well as tumors of neuroendocrine origin. In the central nervous system, somatostatin acts as a neurotransmitter. Outside of the brain, it inhibits the release of growth hormone, insulin, glucagon, gastrin, serotonin, and calcitonin. It also inhibits angiogenesis, is involved in the immune function of leukocytes, and has an antiproliferative effect on tumors.
Five different subtypes of human SSTRs have been identified, expressed to varying degrees on different tumors. Therapeutic drugs have been developed that readily bind to these receptors. Octreotide (Sandostatin) and lanreotide (Somatuline) are somatostatin analogs used clinically to inhibit growth in acromegaly and to control symptoms of carcinoid syndrome. Radiopharmaceuticals have also been developed that bind to SSTRs.
Diagnostic SSTR Radiopharmaceuticals
Indium-111 Pentetreotide (OctreoScan)
OctreoScan was approved by the U.S. Food and Drug Administration (FDA) in 1994 for imaging of NETs. This radiolabeled SSTR binding agent has high affinity for subtypes 2 and 5 ( Fig. 13.2 ). It has high-energy emissions (171, 245 keV), relatively slow pharmacokinetics, and thus unfavorable dosimetry (see Appendix 1 ), which limits the administered dose to 6 mCi (222 MBq) and adversely affects image quality.

Normal Distribution
Splenic and renal uptake is quite high. Lesser uptake is seen in the liver. Low-level hepatobiliary excretion increases intestinal clearance over time ( Fig. 13.3 ). The kidneys rapidly excrete the radiopharmaceutical, with 85% of the dose cleared from the body by 24 hours after injection. Uptake may be seen in the pancreas in the region of the uncinate process; this must not be confused with tumor. Radiotracer uptake is also be seen with benign inflammatory conditions (e.g., thyroiditis, granulomatous disease, inflammatory bowel disease, postradiation therapy, and at sites of recent surgery) due to the SSTRs present on human immune cells (e.g., mononuclear leukocytes, peripheral blood lymphocytes, and macrophages).

Methodology
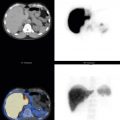
An imaging protocol is described in Box 13.2 . Early planar imaging at 4 hours after injection permits visualization of tumor uptake before bowel excretion; however, 24-hour imaging has a higher tumor-to-background ratio and is more sensitive for tumor detection ( Fig. 13.4 ). Delayed imaging at 48 hours can further confirm tumor uptake versus bowel activity. With single-photon emission computed tomography with computed tomography (SPECT/CT), imaging only at 24 hours is routine, and it improves tumor identification and localization ( Fig. 13.5 ).
Patient Preparation
None
Radiopharmaceutical
Children: 0.14 mCi/kg (5 MBq/kg)
Adults: 6 mCi (222 MBq) In-111 octreotide, intravenously
Instrumentation
Gamma camera: Large field of view
Collimator: Medium energy
Windows: 20% centered at 173 keV and 247 keV
Acquisition
Imaging: Planar whole body and SPECT or SPECT/CT of abdomen and or other indicated site at 24 hours
If only planar imaging: 4 and 24 hours; 48-hour images may occasionally be useful
Whole-Body Images
Dual-head camera 6 cm/min (approximately 40 minutes head to below hips)
1024 × 512 word matrix
SPECT
128 × 128 matrix, 3-degree angular sampling, 360-degree rotation, 35 sec/stop
Fusion to CT or SPECT/CT preferable
CT, Computed tomography; SPECT, single-photon emission computed tomography; SPECT/CT, single-photon emission computed tomography with computed tomography.


Accuracy
The sensitivity of In-111 pentetreotide for tumor detection is reported to be high for carcinoid tumors (85%–95%; Box 13.3 ). However, a lower sensitivity of approximately 75% occurs with pancreatic NETs (e.g., gastrinomas, glucagonomas, vasoactive intestinal polypeptide-secreting tumors [VIPomas], and nonfunctioning islet cell tumors). Because of high normal liver uptake, liver metastases are not always detected. Detection may be reduced in patients on octreotide therapy; thus, the study should be performed immediately before the patient’s monthly therapeutic injection.
High
Carcinoid (86–95%)
Islet cell tumors (75–100%)
Gastrinoma, glucagonoma, VIPoma
Adrenal medullary tumors (>85%)
Pheochromocytoma, neuroblastoma, paragangliomas
Small-cell lung cancer (80–100%)
Moderate
Medullary thyroid (50–75%)
Insulinoma (25–70%)
Medulloblastoma (61–93%)
Meningioma (50% and 100% reported)
Low
Pituitary adenoma
Astrocytoma grade IV (higher in grades I and II)
Breast cancer
Melanoma
Renal cell carcinoma
VIPoma, Vasoactive intestinal polypeptide-secreting tumor.
Gallium-68 (Ga-68) Dotatate (DOTA-0-Tyr3-Octreotate )
Several Ga-68-labeled somatostatin receptor positron emission tomography (PET) imaging agents have been investigated, including Ga-68 dotatoc, dotanoc, and dotatate (see Fig. 13.2 ). These short amino acid–chelator conjugates demonstrate superior affinity for somatostatin receptors compared with In-111 pentetreotide. The three dota agents are similar in imaging accuracy. Ga-68 dotatate (NetSpot) was approved by the FDA in 2016 for imaging of neuroendocrine tumors. The radionuclide, Ga-68, is produced in a Germanium 68/Ga-68 generator, similar to a Molybdenum-99/Tc-99m generator. The parent Ge-68 has a half-life of 271 days; thus, the generator can be used for at least a year. The Ga-68 daughter has a half-life of 68 minutes. In a high-volume clinic, a generator can make sense. Alternatively, some commercial radiopharmacies maintain a generator and distribute individual radiopharmaceutical doses on a regional basis. The recommended dose is 0.054 mCi/kg (2 MBq/kg) up to 5.4 mCi (200 MBq).
Normal Distribution
Ga-68 dotatate binds with high affinity to SSTR-2 receptors. Uptake is seen in the pituitary, thyroid, and salivary glands; spleen; adrenals; kidney; prostate; and liver ( Fig. 13.6 ). Normal uptake may also be seen in the uncinate process, similar to that seen with In-111 pentetreotide. There is no brain uptake, but focal activity in the nonenlarged pituitary is normal. Cardiac uptake is absent, and lung uptake is low. Twelve percent of the administered dose is excreted in the urine by 4 hours postinjection.

Methodology
A Ga-68 dotatate PET/CT study requires considerably less patient time than SPECT/CT In-111 pentetreotide. PET/CT imaging begins approximately 1 hour after injection of the radiopharmaceutical. The total time from injection to the completion of imaging is about 2 hours, similar to routine F-18 FDG oncologic imaging. The radiation dose to the patient is less with Ga-68 dotatate compared with In-111 pentetreotide (see the Appendix).
Accuracy
In a large retrospective study of 728 patients with NETs, Ga-68 dotatate had a sensitivity of 94% and a specificity of 92%. The highest accuracy was for primary midgut tumors. In a comparison study of 131 patients with NETs and unknown primaries, Ga-68 dotatate PET/CT had a higher detection rate (95%) compared with In-111 pentetreotide SPECT/CT (31%) or CT or MRI (46%) ( Fig. 13.7 ). Additional clinical information is found in 70% to 80% of Ga-68 dotatate cases compared with pentetreotide (OctreoScan), and a change in clinical management is reported to occur in greater than 40% of patients ( Figs. 13.8 and 13.9 ). Patients with poorly differentiated tumors may not have uptake and may benefit from F-18 FDG PET, although these patients have a poorer prognosis. The use of intravenous contrast with SSTR PET/CT increases the detection rate for liver metastases and small bowel primaries. SSTR PET/MRI is also reported to provide improved detection of liver metastases.



Other Neuroendocrine Tumors
Because neuroendocrine cells are spread throughout the body, NETs can develop in many different places, including in endocrine glands. Other NETs include medullary carcinoma that starts in the C cells of the thyroid, parathyroid carcinoma or parathyroid adenoma, thymic neuroendocrine cancer, pheochromocytoma that starts in the chromaffin cells of the adrenal glands, paraganglioma, neuroblastoma, pituitary gland tumors, neuroendocrine tumors of the ovaries and testicles, Merkel cell carcinoma, a type of nonmelanoma skin cancer, and small-cell lung cancer.
Therapeutic Radionuclides Bound to SSTRs
High doses of In-111 pentetreotide have been investigated for peptide receptor radionuclide therapy (PRRT) of NETs, taking advantage of the radionuclide’s Auger and conversion electron emissions. Although showing effectiveness, more promising results were found using a pure beta emitter, Yttrium-90 (Y-90), bound to other SSTRs, dotatoc and dotatate. In spite of increased survival, bone marrow and renal toxicity were a significant problem.
Lutecium-177 (Lu-177) Dotatate (Lutathera) Therapy for Neuroendocrine Tumors
As an alternative to Y-90, Lu-177 labeled to dotatate, a beta and gamma emitter, was investigated. Retrospective studies had shown similar effectiveness without the side effects of Y-90. In 2017 a phase 3 multicenter randomized prospective controlled trial of Lu-177 dotatate ( Fig. 13.10 ) was published that studied therapeutic effectiveness in patients with advanced midgut (jejunum, ileum, proximal colon) NETs who had disease progression while on standard first-line octreotide therapy. The investigation reported that compared with octreotide therapy, Lu-177 dotatate resulted in longer progression-free survival (65% vs.11% at 20 months), a significantly higher response rate (18% vs. 3%), and a lower risk of progression, 79% lower than high-dose octreotide therapy. Clinically significant myelosuppression occurred in <10% of patients. Risk of death was 60% lower. Renal toxicity was not a problem with amino acid infusion. Lutathera was approved for clinical use by the FDA in early 2018.

Methodology
Lu-177 dotatate, 200 mCi (7.4 MBq), is infused intravenously over 30 minutes. Patients receive at least four infusions at 8-week intervals. The amino acid solution is infused simultaneously for renal protection. However, significant nausea accompanies the amino acid solution and requires simultaneous antinausea therapy. Reports suggest that the combination of lysine and arginine has far fewer side effects than the amino acid solution.
F-18 Fluorodeoxyglucose PET/CT
The sensitivity of F-18 FDG PET/CT for tumor detection is low in well-differentiated NETs. However, FDG PET has an important role in the imaging of aggressive, poorly differentiated tumors. If Ga-68 dotatate is negative, FDG imaging should be performed. Some advocate using both because tumors can be quite heterogeneous.
I-123 and I-131 mIBG Adrenergic Tumor Imaging and Therapy
Radiolabeled metaiodo-benzyl-guanidine (mIBG) adrenal medullary scintigraphy has been used clinically since the 1980s for diagnosis and staging of neural crest tumors (e.g., pheochromocytomas, paragangliomas, and neuroblastomas). I-131-labeled mIBG was the original diagnostic agent; however, I-123-labeled mIBG is now widely available and preferable because of its superior image quality with an optimal 159-keV gamma-ray energy and lower patient radiation given a lack of β – emissions and shorter half-life of 13 hours as opposed to I-131 with a 364-keV gamma-ray energy, β – emissions, and 8-day half-life (see Appendix 1 ). I-131 mIBG is reserved for therapy.
I-123 mIBG (AdreView)
I-123 mIBG (AdreView) was approved by the FDA in 2003 for the detection of primary or metastatic pheochromocytoma and neuroblastoma, as an adjunct to other diagnostic tests. As an analog of the drugs bretylium and guanethidine, mIBG shares structural features and biological behavior with the adrenergic neurotransmitter hormone norepinephrine. Both norepinephrine and mIBG are taken up in cells rich in sympathetic neurons by an active process mediated by the norepinephrine uptake-1 transporter. Once in the cytoplasm, it is actively transported into the presynaptic nerve terminal and catecholamine storage granules by the vesicular monoamine transporter ( Fig. 13.11 ).

Uptake and Distribution
I-123 mIBG avidly localizes in organs with high adrenergic innervation, including the heart, salivary glands, kidneys, and liver. ( Fig. 13.12 ). Variable activity is seen in the lungs, gallbladder, salivary glands, and nasal mucosa. Mild to moderate adrenal uptake often occurs with planar I-123 mIBG imaging and is nearly always seen with SPECT. No uptake occurs in the normal skeleton. It is cleared through the colon and kidneys.

Methodology
Numerous drugs interfere with mIBG uptake. The most common include tricyclic antidepressants, reserpine, cocaine, and the alpha- and beta-blocker labetalol ( Table 13.3 ). A detailed imaging protocol is summarized ( Box 13.4 ). Pretreatment with saturated potassium iodide (SSKI) or Lugol’s solution is recommended in the package insert and in procedural guidelines to block thyroid uptake ( Table 13.4 ). Although the 159-keV photopeak can be imaged with a low-energy collimator, a fraction of the photons (<3%) are high energy (440–625 keV [2.4%] and 625–784 keV [0.15%]), reducing the image quality. Although a low-energy collimator is often used, a medium-energy collimator is preferable. Images are routinely acquired 24 hours after injection. Whole-body imaging is standard in order to detect an extraadrenal pheochromocytoma, malignant metastases, or primary and metastatic neuroblastoma. SPECT/CT is very useful for anatomical localization.
| Drug | Related Drugs | Mechanism | Discontinue for: |
|---|---|---|---|
| Antihypertensive/cardiac agents | Bretylium, guanethidine, reserpine Calcium channel blockers (amlodipine, nifedipine, nicardipine) Labetalol | Deplete granules Deplete granules Deplete granules and inhibit uptake Beta-blocker | 7 days 14 days 21 days |
| Antipsychotics | Butyrophenones (droperidol, haloperidol) Loxapine Phenothiazines (chlorpromazine, fluphenazine, promethazine) | Inhibit uptake Inhibit uptake Inhibit uptake | 21 days |
| Cocaine/opioids | Inhibit uptake | 7 days | |
| Sympathomimetics | Amphetamine, dopamine, ephedrine, isoproterenol, fenoterol, phenylephrine, phenylpropanolamine, pseudoephedrine, salbutamol, terbutaline, xylometazoline | Deplete granules | 7 days |
| Tramadol | Inhibits uptake | 14 days | |
| Tricyclic antidepressants | Amitriptyline (and derivatives), amoxapine, doxepin | Inhibit uptake | 21 days |
Patient Preparation
Discontinue interfering medications ( Table 13.3 ).
Potassium iodide or Lugol’s solution to prevent thyroid uptake ( Table 13.4 )
Radiopharmaceutical
Intravenous injection over 30 seconds
I-123 MIBG
Children: 0.14 mCi/kg (5.2 MBq/kg); minimum 1.0 mCi (20 MBq) and maximum 10 mCi (400 MBq)
Adults: 10 mCi (400 MBq)
Instrumentation
Gamma camera: Large field of view for planar images
Planar imaging and SPECT/CT as indicated
Collimator: Medium energy, parallel hole; low energy can be used.
Acquisition
I-123: Image at 24 hours.
Whole-body planar images (8 cm/sec)
SPECT: 3-degree steps, 35 sec/step, 180 projections, 128 × 128 matrix
mIBG, Metaiodo-benzyl-guanidine; SPECT, single-photon emission computed tomography; SPECT/CT, single-photon emission computed tomography with computed tomography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree