Abstract
The current field of open fetal surgery is the result of superior diagnostic imaging, comprehensive multidisciplinary teamwork, and ongoing research endeavors. Prenatal indications for fetal surgery continue to expand, and today’s fetal surgery leaders are moving toward alternative minimally invasive methods to reduce surgical risk to the maternal-fetal dyad. In this chapter we briefly discuss the history of open fetal surgery, highlight current indications for fetal surgical intervention, and present our program’s systematic approach to open fetal surgery.
Keywords
open fetal surgery, myelomeningocele, fetal anesthesia
Introduction
Development of fetal surgery would not have been possible without advances in prenatal imaging, understanding the natural history of diseases, experimental animal models, and above all, the vision, innovation, and discipline of a dedicated group of surgeons, maternal-fetal medicine specialists, and anesthesiologists. The first human open fetal intervention was performed in 1982, with bilateral ureterostomies to manage a lower urinary tract obstruction (LUTO). Fetal surgery poses a unique challenge as it involves two patients: the mother and her fetus, a scenario similar to transplantation from a living donor. Because of the risk to the mother who derives no direct benefit from fetal surgery, open fetal surgery was only performed in lethal conditions. In the intervening decades, however, the indications have been expanded to include non–life threatening conditions such as myelomeningocele (MMC). Before human implementation, surgical, tocolytic, and anesthetic techniques were developed and refined in sheep and nonhuman primate models with extensive translational research. Innovative techniques for opening and closing the gravid uterus had to be developed to minimize risks to the mother’s health and future reproductive potential, including the development of an absorbable uterine stapler to minimize myometrial bleeding.
Every fetal intervention requires a rigorous process entailing understanding the natural history of the disease, demonstrating its pathophysiology, and providing proof of concept in an applicable animal model before any human surgery ( Table 118.1 ).
|
In this chapter, we will focus on the techniques of open fetal surgery, anesthetic management, and intraoperative monitoring as applied for MMC with a brief synopsis on the other indications ( Table 118.2 ).
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Current Indications for Open Fetal Surgery
Myelomeningocele
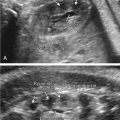
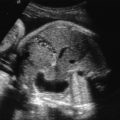
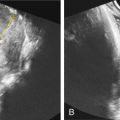
Neural tube defects (NTDs) are the leading cause of central nervous system malformation in humans and a devastating birth defect resulting in destruction of the exposed developing spinal cord ( Chapter 41 ) ( Fig. 118.1 ). The sequelae of NTDs include anatomic effects secondary to the primary defect, as well as functional, emotional, and psychologic morbidities including bladder and bowel incontinence, paralysis, musculoskeletal deformity, shunt malfunctions, and infections. The associated morbidity and mortality is thought to be a result of prolonged mechanical trauma to neural elements, as well as chemical irritation and inflammation associated with exposure to toxic molecules in the amniotic fluid ( Fig. 118.2 ). Ultimately, the compound nature of this malformation results in an immense financial burden on the afflicted families and our health care system amounting to $1,400,000 per child affected by MMC, over a 20-year life span. The incidence has plateaued around 1 in 1000 births following an initial decrease from widespread prenatal folic acid supplementation. With a global population of 7 billion and a yearly birth rate of 20 per 1000 individuals, there are about 140,000 NTD cases per year worldwide ( Fig. 118.3 ).



In animal models, prenatal coverage of NTDs preserves neurologic function and improves hindbrain herniation. In addition, naturally occurring skin-covered NTDs are usually neurologically intact without the complications of open NTDs, and ultrasound (US) and autopsy studies have demonstrated that injury to the exposed neural tissues is a progressive process. Prenatal repair of open spina bifida is performed at 23–24 weeks and is associated with a 50% reduction in the need for postnatal ventriculoperitoneal (VP) shunting as well as a higher likelihood of ambulation. The Management of Myelomeningocele Study (MOMS) trial hinges on achieving skin closure of the defect to prevent further neural damage and cerebrospinal fluid leak. Table 118.2 summarizes the inclusion and exclusion criteria for offering prenatal repair of MMC according to the MOMS trial.
Our group has extended the inclusion criteria for offering prenatal repair of MMC to include maternal body mass index >35. More recent follow-up results from the MOMS trial show that the size of the fetal lateral ventricles at the time of prenatal MMC repair determines the likelihood that VP shunting can be avoided. In addition, even though repair with dilated lateral ventricles (≥15 mm) may not prevent the need for VP shunting, prenatal repair may improve the neurologic function of lower extremities and decrease the need for chronic intermittent bladder catheterization.
Fetal Resection for Large Congenital Pulmonary Airway Malformation Lesions
Congenital cystic adenomatoid malformation (CCAM) also recently known as congenital pulmonary airway malformation (CPAM) is a benign congenital cystic lung lesion of pulmonary tissue with proliferation of bronchial structures ( Chapter 2 ). It was originally described as an adenomatoid proliferation of the terminal bronchial tree resulting in cystic lesions coated in bronchial epithelium ( Figs. 118.4 and 118.5 ).


Fetuses found to have a fetal chest mass on a screening US should undergo a comprehensive evaluation at a tertiary care center including ultrasonography, ultrafast magnetic resonance imaging (MRI), and fetal echocardiography. The differential diagnosis of a fetal chest mass includes CCAM, bronchopulmonary sequestration (BPS), bronchogenic cyst, and congenital diaphragmatic hernia. CPAMs occur at the fifth or sixth week of gestation during the pseudoglandular stage of lung development but do not tend to grow rapidly until 20 weeks’ gestation. Their classification has been modified by Adzick et al. depending on their natural history and effects to include either microcystic or macrocystic lesions. The natural history of prenatally diagnosed lung lesions is variable and depends on their size and resulting physiologic derangements. Almost 20% of fetal CCAMs demonstrate a decrease in size, and two-thirds of BPS lesions shrink dramatically before birth. Because of this unpredictable growth, we recommend biweekly US evaluations until growth plateau is achieved. To better understand the natural history and prognosticate CPAM lesions, we developed the CPAM-Volume-Ratio (CVR). A CPAM volume is determined using the formula for a prolate ellipse (length × height × width × 0.52). The CVR is obtained by dividing the CPAM volume by head circumference to correct for differences in fetal size. A CVR >1.6 at the initial presentation is predictive of an 80% risk of developing hydrops. The development of fetal hydrops unresponsive to maternal steroid administration is the only indication for fetal intervention in fetal lung lesions, as it may be an indicator of impending fetal demise. Maternal betamethasone was empirically observed to arrest the growth of CCAMs and allow the fetus to grow around the CPAM and cause hydrops to resolve.
Open fetal surgery should be considered in predominantly solid large lung lesions associated with fetal hydrops and mediastinal shift, presenting before 32 weeks’ gestation that have not responded to at least one or two courses of maternal betamethasone.
Fetal Debulking for Large Sacrococcygeal Teratomas
Sacrococcygeal teratoma (SCT) is a congenital germ cell tumor. It is a mass located at the base of the coccyx and occurs in an average of 1 : 40,000 births. Most newborns diagnosed with SCT have a very favorable outcome, with a very small risk of associated malignancy. In contrast, prenatally diagnosed SCT has a poor prognosis and is fatal 30% to 50% of the time. These tumors have a variable consistency, can undergo an unpredictable rate of growth, and demonstrate significant hemodynamic changes. While US is the primary modality to diagnose SCT ( Fig. 118.6 ), MRI is crucial to delineate the intrapelvic extent of the SCT ( Fig. 118.7 ). The high mortality associated with prenatally diagnosed SCTs may be caused by premature birth secondary to polyhydramnios, dystocia secondary to large tumors resulting in traumatic tumor rupture and hemorrhage, and high output cardiac failure as a result of a large blood vessel supplying the tumor, or hemorrhage into the tumor. A large tumor may result in a vascular steal from the placenta that can be documented by Doppler US and echocardiography. Some of the associated manifestations include reversal of diastolic flow in the umbilical arteries, abnormalities of placental thickness, diameter of the suprahepatic inferior vena cava, combined ventricular output, and descending aortic flow velocity. The end result of this is placentomegaly, fetal hydrops, and ultimately a maternal “mirror syndrome,” which can be life threatening for both mother and fetus. Very similar to cases of CCAM with hydrops, fetal intervention in these cases is indicated before the development of maternal mirror syndrome, at which point the only option is delivery.