Fig. 5.1
Molecular imaging of endothelial VCAM-1 expression. A VCAM-1-targeted NIRF probe shows co-localization with VCAM-1 staining by immunohistochemistry in mouse aorta. This signal is also associated with CD31 expression, indicating the presence of endothelium, and Mac-3 expression that reveals underlying macrophages. Original magnification ×400. Bar = 50 μm (Figure is courtesy of Dr. M. Nahrendorf)
Following adhesion to the endothelial cells and migration from the lumen to the subendothelial space, monocytes give rise to macrophages that play a central role in the growth of the neointimal atherosclerotic plaque. Scavenger receptors on the plasma membrane allow these macrophages to phagocytose, or engulf, cholesterol within the early lesion forming foam cells that serve as a means of cholesterol clearance from the plaque. Molecular probes targeting macrophage scavenger receptors have shown the ability to detect inflammation by accumulating in macrophage-rich atherosclerotic plaques of mice with the amount of detectable signal proportional to the degree of macrophage number [24]. Similarly, due to their phagocytic action, dextran-coated magnetofluorescent nanoparticles were shown to exhibit the highest uptake in plaque macrophages compared to other cell types within the plaque [25]. The specificity of these nanoparticles can be further enhanced using ligands to known macrophage markers.
Inefficiency in removing apoptotic foam cells leads to the formation of a necrotic core within the plaque that has been shown to be associated with plaque destabilization. Therefore, the identification of macrophage apoptosis within lesions would allow the detection of potentially vulnerable plaques prior to rupture. High-density lipoprotein (HDL) mimicking polymer nanoparticles with a quantum dot core and a cationic ligand that targets the mitochondria of apoptotic cells and a quantum dot core were recently developed [26]. The fluorescent quantum dots allowed optical imaging of macrophage apoptosis in vitro as the nanoparticles accumulated in the mitochondria of dying cells. Elevated plasma HDL has been shown to reduce cardiovascular risk by promoting reverse cholesterol transport. In a mouse model, injection of the HDL-mimicking nanoparticles led to a reduction in total serum cholesterol in the mice. These results demonstrate the combined therapeutic and diagnostic (“theranostic”) potential of agents designed with molecular imaging components. Further, nanoparticle-based imaging strategies provide an efficient means of targeting ligands and imaging probes to the atherosclerotic plaque [27].
5.4 Proteolytic Activity and Matrix Remodeling
In addition to – and perhaps more importantly than – their role in plaque destabilization associated with necrotic core formation, macrophages within the plaque release proteases that degrade extracellular matrix components, directly compromising the structural integrity of the plaque [28]. Increased protease activity due to macrophage accumulation has been shown to lead to a reduction of collagen within the atherosclerotic fibrous cap [1]. Thin fibrous cap atheroma is not able to withstand the stresses caused by hemodynamic shear and systolic pressure, and biomechanical failure of the cap leads to the formation of thrombi causing vessel occlusion that results in myocardial infarct and stroke. Therefore, early detection of matrix degradation due to the presence of proteases within the plaque represents an attractive target for molecular imaging probes. Researchers have taken advantage of the enzymatic activity and extracellular localization of these proteases to develop compounds that exhibit NIRF signal following proteolytic activation.
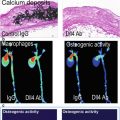
Cysteine protease activity was the target of the first generation of enzymatically activated NIRF molecular probe agents [29, 30]. These probes were synthesized by incorporating NIRF molecules on cysteine peptide chains. The close proximity of the NIRF agents quenches the fluorescent signal due to fluorescence resonance energy transfer. Cleavage of the chains by cysteine proteases liberates the NIRF molecules, leading to observable fluorescent signal. Cysteine proteases include members of the cathepsin family of enzymes, namely, cathepsins B, K, and S that are the most relevant targets for atherosclerosis [30, 31]. An activatable cathepsin B-targeted NIRF agent shows lysosomal uptake and accumulation in plaque inflammatory cells [30]. Similarly, catheter-based intravascular imaging of cysteine protease activity following injection of these agents in a rabbit model of arteriosclerosis revealed strong NIRF signal in inflamed plaques that was associated with cathepsin B activity [32]. Incorporating peptide sequences targeted to enzymes of interest (rather than the nonspecific cysteine peptide chain) enables the detection of specific proteases within the plaque and can give insight into biomolecular mechanisms of plaque development. Using this strategy, cathepsin S, an elastolytic cysteine protease, was found to be increased in atherosclerotic plaques of a mouse model of chronic renal disease (CRD) with a strong association to calcification [33] (Fig. 5.2a). Osteogenic activity and mineral deposition were abolished in mice lacking cathepsin S activity, demonstrating a causative link between cathepsin S and calcification (Fig. 5.2b).
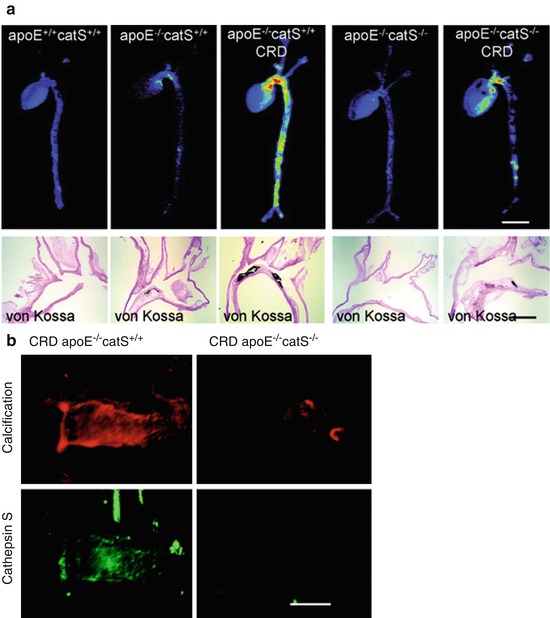

Fig. 5.2
Molecular imaging of cathepsin S and calcification. (a) Fluorescence reflection imaging of a NIRF probe targeted to cathepsin S revealed the highest cardiovascular activity in an ApoE−/− mouse model of chronic renal disease (CRD). This increased activity was also associated with aortic calcification as identified by von Kossa staining of histological sections. (b) Using the NIRF cathepsin S probe in combination with a NIRF probe for calcification showed a strong association between the two. Further, by knocking out cathepsin S in the mice, calcification of the aorta was mitigated (Modified from Aikawa et al. [33])
In a similar manner to the probe for cathepsin S, second-generation activatable NIRF agents were developed to measure collagen-degrading matrix metalloprotease (MMP) activity by replacing the cysteine chain with a gelatinase substrate (collagenase MMPs also recognize gelatin as a substrate) [34]. An atherosclerotic mouse model on a high-cholesterol diet was given injection of the MMP-sensitive NIRF agent 24 h prior to imaging with fluorescence-mediated tomography revealing significant signal in the aortic root, arch, and thoracic aorta corresponding to sites of atheroma. These agents may provide a means for early identification of particularly vulnerable plaques. MMPs have been shown to mediate collagen degradation within the atherosclerotic fibrous cap, leading to diminished biomechanical integrity, plaque rupture, and thrombosis. Therefore, imaging MMP activity in early atheromas could allow clinicians to identify areas of concern prior to rupture.
5.5 Inflammation, Osteogenesis, and Calcification
In addition to their participation in plaque remodeling via the release of matrix-degrading enzymes, inflammatory cells also contribute to the formation of calcific deposits within the plaque (Fig. 5.3). In vitro studies have demonstrated that macrophage-derived cytokines (e.g., IL-1β, IL-6, IL-8, TNF-α, IGF-1, and TGF-β) can induce osteogenic differentiation of vascular SMC [7, 8]. Evidence also suggests that macrophages can actively participate in the nucleation of calcium phosphate mineral [6]. Imaging mineral deposition by macrophages and SMC in the vessel wall has been achieved using bisphosphonates conjugated to NIRF molecules [35]. Bisphosphonates have a structure similar to the calcification inhibitor pyrophosphate and are believed to bind to calcium and accumulate in mineralized crystals [35, 36]. The accumulation of NIRF-bisphosphonates in calcific regions of atherosclerotic plaques yields detectable NIRF signal that is spectrally distinct from the protease agents discussed in the previous section [37]. Imaging these processes in parallel has provided new insight into the relationship between inflammation and calcification [5, 38].
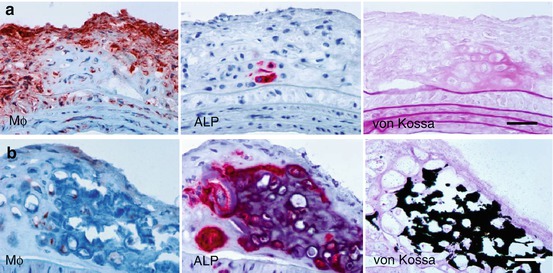

Fig. 5.3
(a) Early osteogenic activity detected by alkaline phosphatase (ALP) in macrophage-rich lesion. (b) Prominent calcification in advances plaque. Cryosections stained with anti-macrophage Mac-3 antibody (left), ALP (middle), and von Kossa (right). Original magnification ×400. Bar = 50 μm (Modified from Aikawa et al. [5])
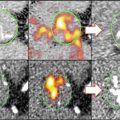
Whether by priming the matrix, stimulating SMC, or actively depositing mineral, inflammation is closely linked with calcification within the atherosclerotic plaque and ultimately to plaque stability [39]. This point was shown in an atherosclerotic mouse model, wherein fluorescence reflection imaging of explanted aortae from the mice revealed a strong association between inflammation and calcification [5] (Fig. 5.4). NIRF signals from both processes were noted in regions associated with high shear stress including the aortic arch and root, the innominate artery, and the carotid bifurcation. The elevated mechanical stress in these regions has been shown to associate with plaque development in both mouse and humans. Longitudinal studies in Apolipoprotein E (ApoE) deficient mice further demonstrated that not only do the inflammation and calcification signals overlap but also the inflammation seemed to necessarily precede calcific mineral deposition [5] (Fig. 5.5). Therefore, it has been proposed that inflammation-dependent calcification in the atherosclerotic plaque can be divided into three phases: initiation, propagation, and end-stage calcification [4]. The initiation phase consists of macrophage infiltration and matrix remodeling. Subsequently, the macrophages promote a pro-osteogenic environment where both SMC and macrophages participate in the propagation of plaque development through the deposition of calcium phosphate mineral. Once the mineralization begins, the final phase, end-stage calcification, progresses quickly and may be an irreversible endpoint of plaque development (Fig. 5.6).
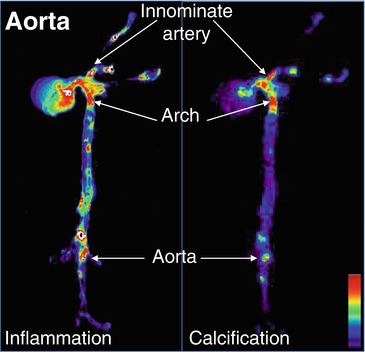
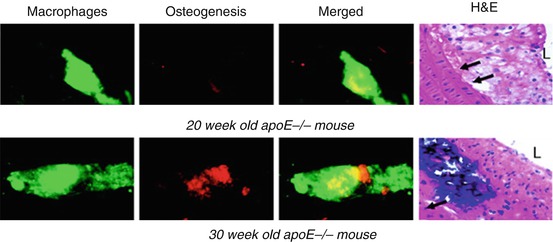
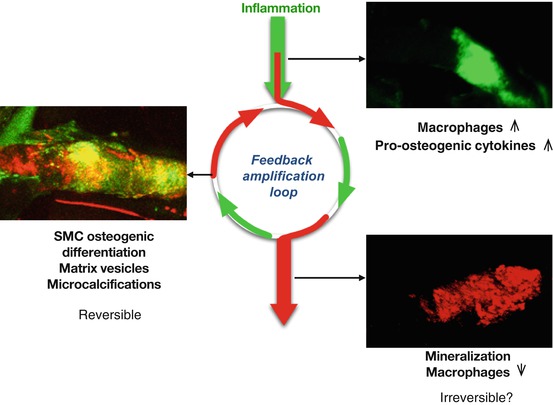

Fig. 5.4
Fluorescence reflection imaging of NIRF-conjugated iron nanoparticles that target macrophages revealed a co-localization between areas of inflammation and calcification in an excised mouse aorta (Modified from Aikawa et al. [5])

Fig. 5.5
Longitudinal study: NIRF probes with different emission wavelengths allow for simultaneous imaging of inflammation (green fluorescence) and calcification (red fluorescence). Using this technique in an ApoE−/− mouse model of atherosclerosis, it was shown that inflammation not only co-localizes with calcification, but also seemed to precede calcific mineral deposition. Twenty-week-old mice exhibited high arterial signal corresponding to the macrophage-targeted probe but did not exhibit signs of calcification. In 30-week-old mice, inflammatory cells and calcification were either within close proximity or co-localized (yellow fluorescence). Representative histomorphological images shown on the right. Hematoxylin and eosin. Magnification ×400. Bar = 50 μm. L depicts lumen; arrows depict internal elastic lamina (Modified from Aikawa et al. [5])

Fig. 5.6
Atherosclerotic plaque development initiates with the infiltration of proinflammatory macrophages that stimulate propagation of plaque development through matrix remodeling and the release of pro-osteogenic cytokines. The initiation and propagation phases may provide the most appropriate window for therapeutic intervention. End-stage calcification may be an irreversible endpoint of plaque development (Figure reprinted from New and Aikawa [4])
5.6 Future Perspectives
As mentioned in the introduction to this chapter, plaque phenotype determines the biomechanical integrity of the atheroma, and both inflammation and calcification play a major role in the biomechanical state of the plaque. The persistence of inflammation in the plaque leads to both heightened protease activity resulting in thinning of collagen in the fibrous cap and the progression of calcification. Further, the presence of macrophages seems to be associated with the formation of microcalcifications within the fibrous cap. Cellular-derived matrix vesicles are believed to serve as nucleating foci for the formation of microcalcifications, and these matrix vesicles may be derived from both the macrophages themselves or from SMC stimulated to undergo osteogenic reprogramming by macrophage releasing proinflammatory cytokines. Though their role in plaque destabilization is now clear, much remains unknown about the mechanisms of microcalcification formation. As we learn more about these mechanisms, new molecular targets for imaging probes may be created to visualize the absolute earliest phases of the calcification process.
Recent advancements using positron emission tomography/CT (PET/CT) with 18F-sodium fluoride (18F-NaF), an established PET tracer for bone formation and remodeling, and 18F-fluorodeoxyglucose (18-FDG), shown to accumulate in regions of inflammation, may provide new strategies for honing in on regions of plaque vulnerability [40]. Coronary uptake of 18F-NaF was found overlaying, adjacent to, and distal from regions of CT-identified calcifications [41]. Additionally, large areas of calcification with no 18F-NaF uptake were observed. This suggests that, as with bone, 18F-NaF uptake in the vasculature is a marker of ongoing calcific remodeling [41]. Large, stable calcifications do not exhibit 18F-NaF update, whereas active regions of mineralization accumulate 18F-NaF. The 18F-NaF regions faraway from the CT-identified calcific regions may represent the dangerous microcalcifications that cannot be detected by traditional imaging modalities [41]. In support of this hypothesis, a prospective clinical trial showed high 18F-NaF accumulation in the culprit coronary plaques in cases of myocardial infarction and in ruptured carotid artery plaques [42]. Histological evaluation of these plaques revealed active calcification processes. These PET/CT techniques exhibit promise in identifying particularly vulnerable regions within the vasculature; however, they still do not have the resolution necessary to identify specific microcalcifications that may contribute to plaque rupture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree