The potential of artificial intelligence (AI) in radiology goes far beyond image analysis. AI can be used to optimize all steps of the radiology workflow by supporting a variety of nondiagnostic tasks, including order entry support, patient scheduling, resource allocation, and improving the radiologist’s workflow. This article discusses several principal directions of using AI algorithms to improve radiological operations and workflow management, with the intention of providing a broader understanding of the value of applying AI in the radiology department.
Key points
- •
Artificial intelligence (AI) can be used not only for diagnostic purposes but also for a variety of operational tasks in the radiology workflow.
- •
Machine learning algorithms can guide workflow optimization by identifying the key factors responsible for workflow outcomes.
- •
Although operational AI tools demonstrate remarkable potential for improving radiology workflow, more work needs to be done to make them widely available.
Introduction
Over the past few years, artificial intelligence (AI) has made a significant advance in the medical world, particularly due to developments in the field of machine learning (ML) and deep learning (DL), coupled with tremendous increases in computational processing power. This has led to the development of new tools enabling computerized image analysis, mainly for tasks of classification and detection of abnormalities. Combining these tools with radiomics (providing quantitative image data not perceptible to the human eye) and data from the electronic medical record (EMR) makes it possible to evaluate patients’ response to therapy, paving the way to personalized treatments.
The potential applications of AI in radiology, however, go well beyond image analysis for diagnostic and prognostic purposes. It is becoming increasingly clear that AI algorithms also can be used for a variety of nondiagnostic tasks to provide support in all levels of the radiology workflow for purposes, such as scheduling, prioritizing, quality, safety, and operational efficiency. , Radiology business intelligence and management tools, providing real-time dashboarding and performing workflow analysis, also can be guided by ML. , Time to image, modality utilization, and report turnaround time (RTAT) are just a few of the metrics that already are utilized. As health care progresses from volume to value, such intelligent enterprise view of the radiology department will be useful to maintain the highest-quality services at the lowest cost.
Although the technology is present and already reported in multiple health care institutions, clinical implementation of AI tools in its broadest sense still is in its early phase and remains rather limited. One of the major reasons is because integration often is hampered by a lack of infrastructure, processes, and tools. Complex radiology information technology (IT) environments pose unique challenges in implementing AI-based solutions. Moreover, AI tools cannot work on their own and need to be properly integrated and maintained. To do so, serious work is needed to ensure the readiness and suitability of the existing informatics infrastructure in the radiology department and in the hospital.
Another reason for the slow-moving implementation of AI in clinical practice can be found in tight policies and regulations, governing the use of AI in clinical environments. AI-based applications for “diagnosis, prevention, monitoring, treatment, or alleviation of disease” belong to the category of Software as a Medical Device (SaMD), as defined by the International Medical Device Regulators Forum (IMDRF). This means that they must meet more stringent requirements and thus also have to go through a longer trajectory before they can be marketed and used effectively in the clinical setting. , The IMDRF also has outlined quality management system principles to systematically evaluate for SaMD applications in terms of their functionalities and performance. Because nondiagnostic AI tools for workflow improvement are not considered as SaMD, there is no regulatory obligation to prove their robustness and validity. It is expected that many of such operational tools will be implemented in the more immediate future.
Financial gains play another important role in determining the applicability of operational AI. With a few exceptions, insurance reimbursements are not currently available in most countries for the use or purchase of AI software. Consequently, the return on investment (ROI) must be evaluated by the demonstration of quality and/or efficiency improvement. For diagnostic-type AI, quality improvement could be achieved by reducing errors or increasing diagnostic accuracy, which by assumption not only could be of clinical benefit but also reduce downstream costs in liability insurance. Demonstrating the ROI for tools that improve efficiency, however, is easier than for tools that improve quality. Reduction of study read times by using diagnostic AI-based software, for example, can be translated directly into person-hours and subsequent cost savings. So, for nondiagnostic applications that in general are more focused on workflow improvement and efficiency, the cost-saving effect is easier to calculate, which also facilitates their implementation. Most of these tools probably will not be sold separately but as an (optional) feature of the vendor’s modality, picture archiving and communication system (PACS), EMR, or speech recognition system, which also facilitates their integration in the existing radiology IT environment.
In this article the authors take a closer look at the types of nondiagnostic AI solutions, which are focused on the automation of operational tasks, such as the evaluation of appropriateness of imaging, patient scheduling, selection of examination protocols, improvement of radiologists’ workflow, and more, to ultimately improve the radiology workflow and patient services ( Fig. 1 ).
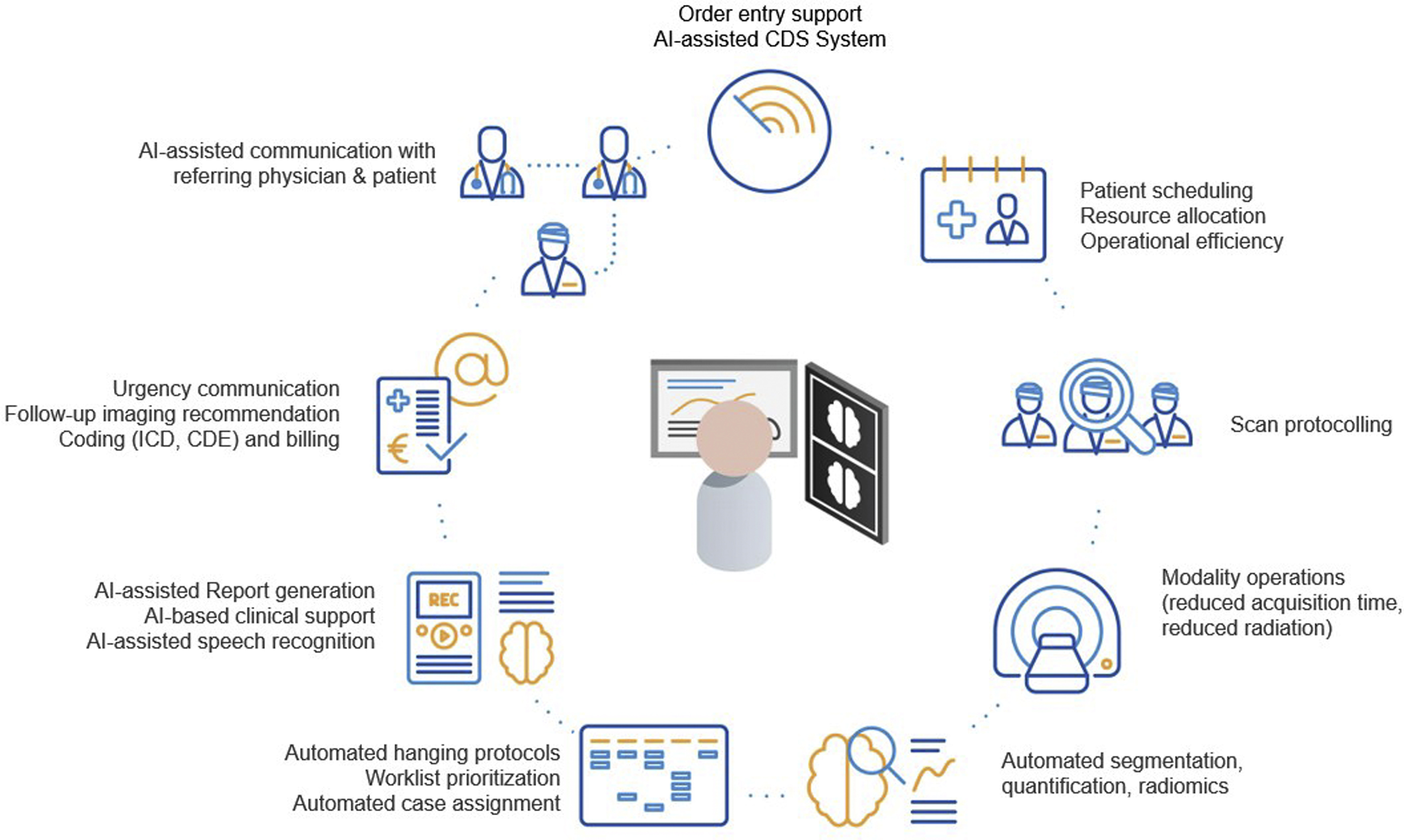
Order entry support
Ordering a patient examination is the first step in the diagnostic imaging chain. It is about not only identifying the right imaging modality to help the patient but also about ensuring the safe execution of the imaging examination or procedure. Over the past few decades, medical imaging utilization has been increasing rapidly. In the United States alone, the number of imaging examinations increased by 26% between 1998 and 2010, mainly due to more advanced types of services, such as computed tomography (CT) and MR imaging. Similarly, a 2018 report by a UK workforce reported a 54% increase in demand for CT examinations and 48% for MR imaging examinations over the previous 5 years, confirming this trend. Due to this relentless increase in workload for radiologists and the associated growing health care costs, continuing with adding more work is not a feasible option. As a result, it is necessary to find solutions to manage this growth while maintaining quality standards of patient care. ,
In the 2 recent decades, in the pre-AI era, several clinical decision support (CDS) systems have been created with the intention of providing referrers with the best possible guidance when ordering imaging examinations, in the belief that this will reduce inappropriate utilization of low-value, high-cost imaging. The Select module of the American College of Radiology (ACR) and the iGuide from the European Society of Radiology (ESR) provide recommendations based on appropriateness criteria and referral guidelines, as defined by dedicated experts and committees. , ACR Select works with a database comprising more than 3000 clinical scenarios and 15,000 criteria. These CDS modules can be incorporated into computerized ordering and EHR systems to guide providers when ordering medical imaging scans. , They calculate an appropriateness score for the requested imaging imagination, based on the clinical information and abnormal prior tests as selected from an examination-specific menu of choices. The benefits of these CDS systems have been documented throughout the literature and are promoted by the ACR and the ESR. A recent trial demonstrated that CDS systems can reduce targeted (ie, likely inappropriate) imaging orders by a statistically significant 6%, allowing reducing imaging-associated costs. The potential of using ML for creating CDS-based order entry support systems nowadays is considered as an important area of research. , Pre-AI CDS systems mostly are developed as rule-based (also known as if-then systems), operating by applying a set of relevant predefined rules and then providing a recommendation. A weakness of such systems is that they become difficult to operate (and even more difficult to maintain) when the number of rules is large or when separate rules contradict each other. In addition, they are able to account for only a limited amount of clinical data as presented in a selection menu, making them impractical for more complex clinical situations. To address these problems, more adaptive data-driven systems can be developed to operate on large data sets through data mining the most appropriate imaging tests by analyzing notes and other EMR features. By integrating ML, the existing shortcomings of CDS systems could be overcome, either by teaching the system much more complex rules or by letting it discover some optimal rules on its own. Such AI-based CDS systems could perform automated screening for renal insufficiency when placing orders for contrast-enhanced studies. Another example is screening for duplicate examination orders within a predetermined timeframe. As was shown by Hassanpour and colleagues, it is possible to develop ML classifiers to analyze narrative radiology reports and to identify high utilizers of imaging services with 94% accuracy. In the future, it should be possible to create a complete digital representation of a patient, a so-called digital twin, allowing a CDS system to make recommendations based on a more holistic approach. , , By combining this in-depth knowledge with adaptive decision-making in complex and uncertain environments, AI-based frameworks will have great value for optimizing radiology examination ordering, reducing overuse of imaging, and erroneous ordering and protocolling of scans. ,
Scheduling and resource allocation
Scheduling and any resource allocation in general represent another recent and interesting development in radiology AI. They also can be viewed as an important direction in health care operations analysis, a relatively new field, aiming at optimizing health care delivery. For years, the clinical aspects of health care were prioritized, looking into better practices and more informative models. Many health care failures, however—delayed or unavailable services, incorrect decisions, and overloaded workflows—have purely operational causes and can have a dramatic impact on the overall quality and safety of patient care. ,
In this regard, resource allocation represents one of the most important and classic problems of workflow optimization ( Fig. 2 ). Each hospital process can be seen as a series of tasks assigned to different resources: for example, specific patient examinations must be scheduled for a particular MRI scanner, depending on the type of examination. Each task can have its own complexity (such as examination duration time or scanning protocol) and each resource its own cost (such as scanner time or negative impact of scanner delays). As a result, for any workflow in question, its resource utilization cost function can be set—for instance, total idle time for all MR imaging scanners in a given outpatient facility. The goal of resource allocation is to compute optimal task-to-resource assignments (schedules), to minimize this predefined cost.
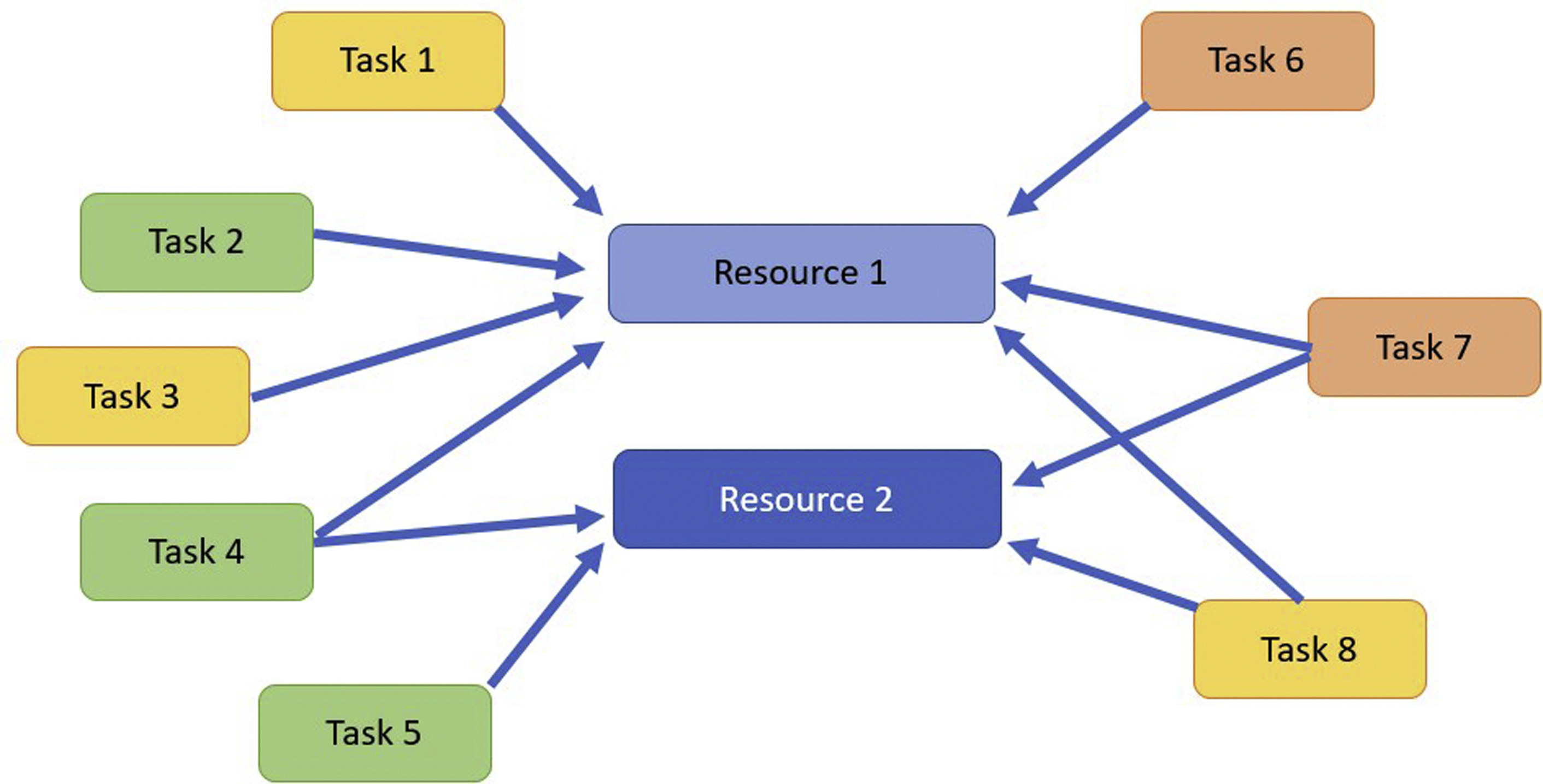
For a single resource or task type (such as scheduling the same MR imaging examination on the same scanner), the problem looks rather trivial and often can be resolved by a human. This virtually never is the case with real-life scheduling, however, where multiple intertwined resources, scheduling constraints, and workflow disruptions make simple solutions impossible. As a result, real operational problems call for more exhaustive, algorithmic solvers.
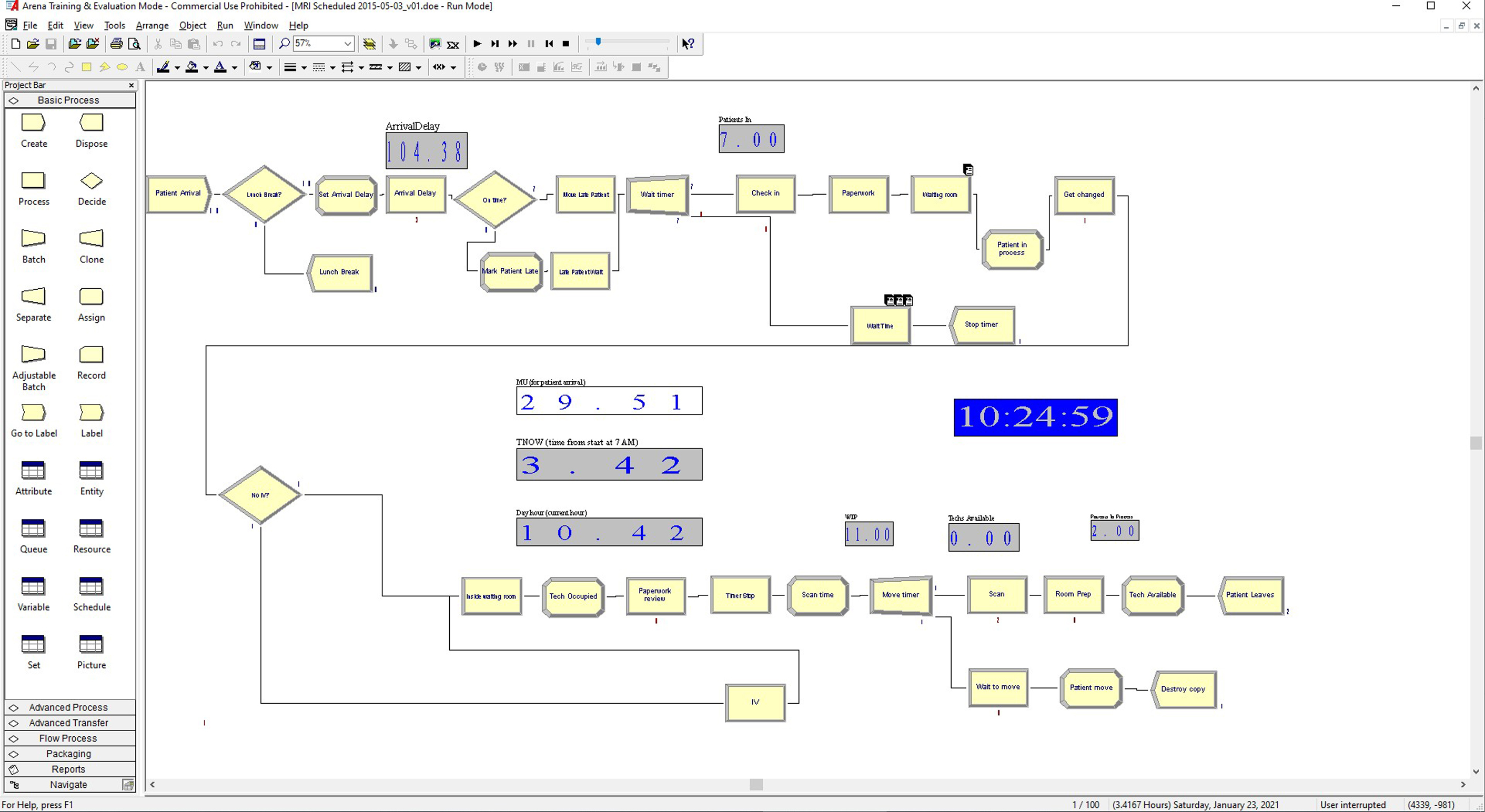
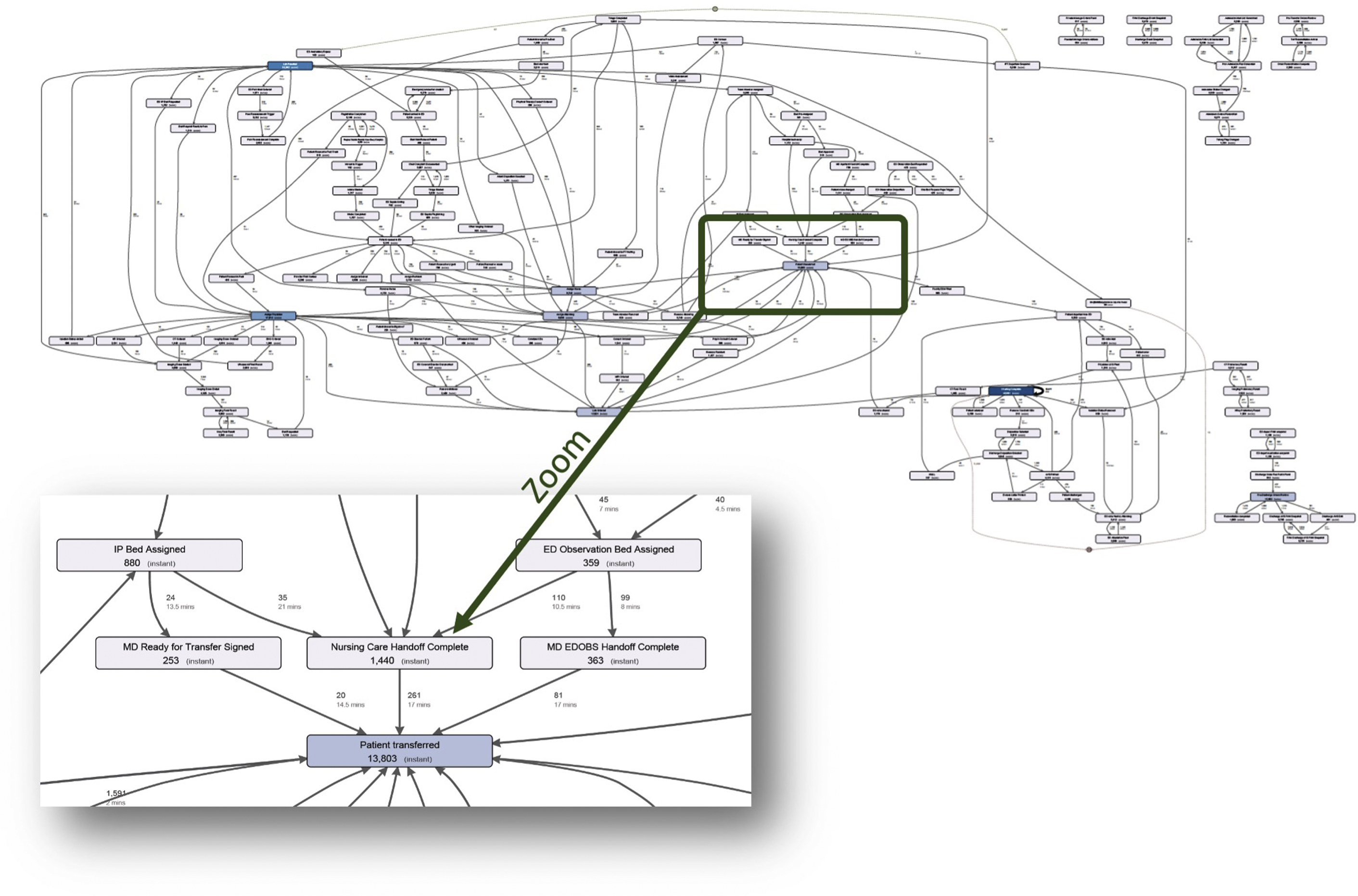
Therefore, many pre-ML scheduling solvers already have been attempted for decades, including linear programming, genetic programming, and branch-and-bound, simulated annealing , as well as more ad hoc algorithms. They were followed by simulation models—discrete event simulations (DESs), in particular—capable of modeling virtually any operational environment. , The entire premise of pre-ML algorithms (such as DES), however, was based on the ability to fully understand the existing environment, and to rewrite it as a logical computer model ( Fig. 3 ).
Although attractive from the interpretability point of view, this workflow model approach ran into a major obstacle: current health care environments could be too complex to interpret. The emergency department (ED) chart in Fig. 4 illustrates this better than anything: it shows the real ED workflow map, which was reconstructed from the actual ED data by a process mining algorithm. When the authors did this for 1 project, it took several weeks to explain it to the emergency physicians, who spent years working in this environment. The main complexity comes from the fact that real health care environments are probabilistic: in a couple minutes, a patient who enters such an environment may end up in any of its branches, with a great degree of probabilistic uncertainty. This probabilistic mess undermines the ability to package the problem into a nice clean flowchart model (such as DES) or define exact relationships among the model parameters (such as required by pre-ML solvers). Therefore, the only way to model this environment is to make the model learn all these complexities on its own, by fitting the model coefficients into the actual workflow data. And this is precisely what ML models are capable of.
First, ML models prove efficient in predicting scheduling and workflow-disrupting events. For example, ML has been explored to predict patient no-shows, negatively affecting both patient care and radiology resource utilization. , , As a result, predicting no-show disruptions with ML can be used for taking practical actions: appointment reminder calls to the patients with the highest predicted no-show risk, double-booking the slots with predicted no-shows, and using these slots for other activities. Similarly, ML models predicting patient risks (implants, adverse contrast reaction, and so forth) could help schedule these patients better and ensure safer scans. Thus, using ML to correctly classify examinations can provide an efficient way for optimizing scheduling.
Second, ML demonstrated good potential in many shared resource problems, when the same resource must be used by multiple concurrent workflows. Waiting rooms in multimodality outpatient centers, recovery rooms in interventional radiology, scanners used by different radiology departments, and more frequently can be shared by radiology and other hospital services and require well-planned scheduling that ML models can create. ,
Another development in radiology ML can be seen in increasing patient-facing AI, created to inform and engage radiology patients. A typical example includes models built to predict patient wait times and facility delays. Although initially designed to inform radiology patients about possible delays, these models proved informative for the radiology management as well—as has been learned, implementing them at the authors’ radiology department. The authors believe that more work will be done in this direction, particularly with using ML models to closely engage radiology patients and address their scheduling needs.
Finally, predicting radiology examination volume offers another example of scheduling problems, aimed at improving radiology planning and finance. For example, the article by Zhang and colleagues provides a detailed treatment of predicting radiology volume in ED and compares multiple ML models as well as identifies the cyclic nature of the model and its features. The study of Guitron and colleagues shows how radiology department volume can be projected accurately a few weeks ahead, even during the uncertain times of pandemic volume drops.
Operational efficiency
The role AI can play in allocating optimal resources immediately points to another important and currently developing direction: identification and removal of hidden bottlenecks in the operational workflow. In an intertwined radiology environment with traffic jams constantly shifting and affecting each other, it is virtually impossible to pinpoint and quantify the best direction for interventions. If an MR imaging outpatient facility runs into severe delays, then should the capacity of the recovery room be increased? Or extra MR imaging technologists be added? Or more patients per hour schedule? Or more MR imaging scanners acquired? When only a couple of possible interventions can be afforded and chosen, their projected gains have to be known.
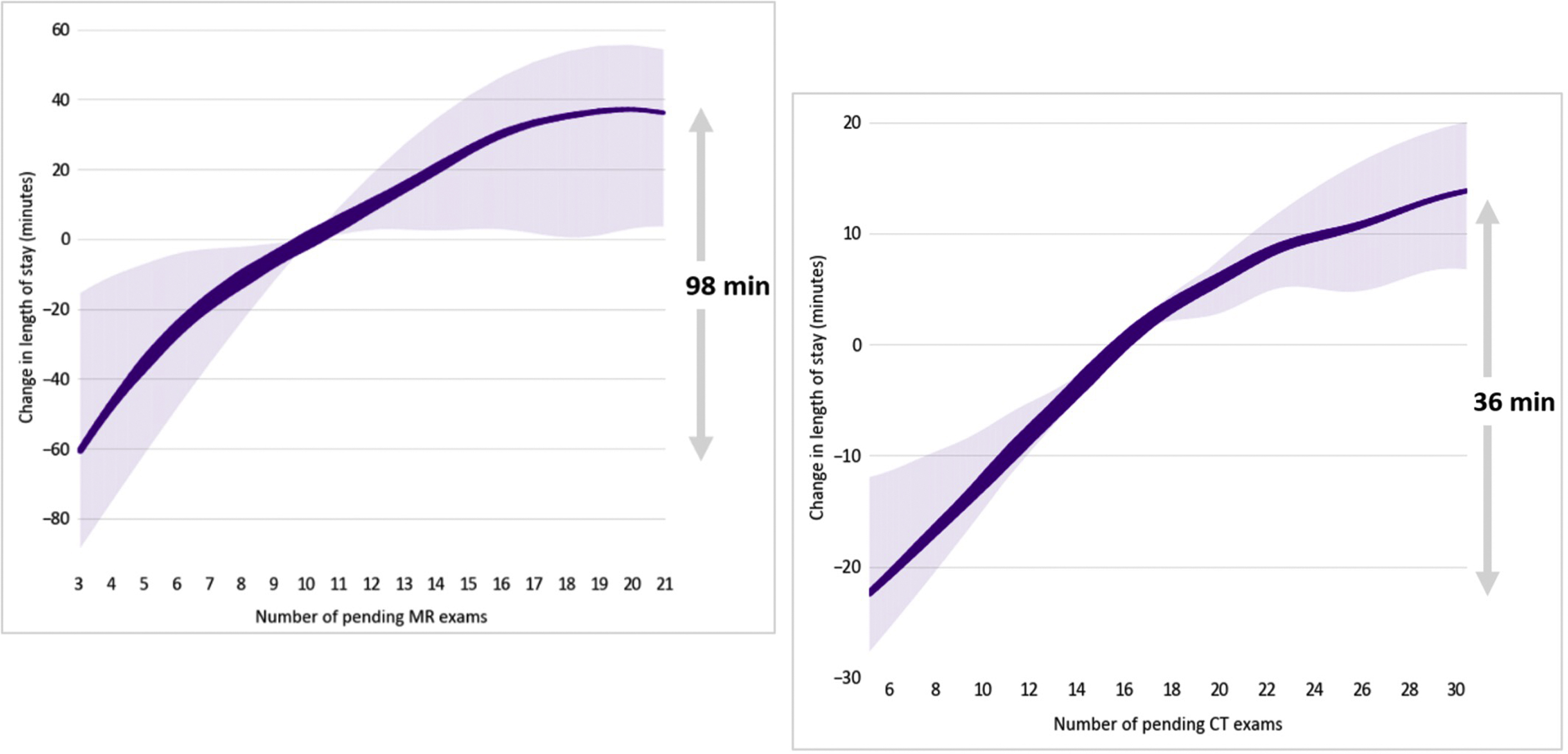
ML-driven workflow optimization solves this problem. This becomes possible because of one of the principal advantages of ML modeling—its built-in ability to detect the best (most predictive) features. This functionality was completely missing in pre-ML models, where one working with unfeasibly exhaustive “what if” model tweaking was possible. Running ML optimal feature selection algorithms—from feature importance to more in-depth feature subset selection—can pinpoint the most critical workflow parameters efficiently and, therefore, suggest the best ways of fixing the problems they are pointing to. For instance, an ML model easily can identify that a certain time of day, or a certain modality queue, is responsible for significant reporting delays, which, if known, subsequently can point to insufficient staffing or overloaded schedule. Moreover, ML models can create partial dependency plots (PDPs) ( Fig. 5 ), identifying the unique contribution of each feature into the main process outcome (such as patient wait time, workflow delay, queueing problem, or no-show). In this way, PDPs can be used to answer many “what if” questions as well but without manual feature exploration, found in pre-ML approaches.