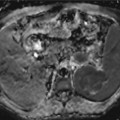
Fig. 42.1
(a–c) Accessory findings of cholangiocarcinoma. (a) Dilatation of biliary tree upstream the lesion, (b) segmental hepatic atrophy, (c) capsular retraction
Biliary tree dilatation upstream the mass is a quite common finding (Fig. 42.1a), as with the enhancement of ductal walls [20]. Dilatation of biliary ducts upstream the mass is quite often an early sign of ICC, even when the mass is still not visible (Fig. 42.2). Sometimes it can be expression of periductal tumoral diffusion (mixed type of ICC) [20] or fibrosis secondary to recurrent cholangitis [21]. Hepatic atrophy is a result of portal vascular encasement [17] associated to biliary obstruction [20]. Lumen stenosis, cutoff, and irregularity of vascular walls are signs of vascular invasion [22].
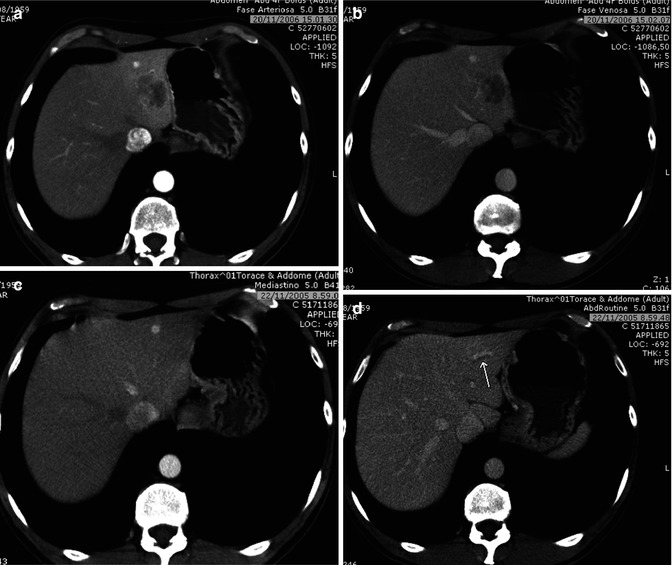

Fig. 42.2
(a–d) Dilatation of biliary ducts as an early sign of ICC. Male, 47-years-old. ICC. (a, b) A hypovascular mass is appreciated in the left lobe; (c, d) CT scan 1 year before. The mass was not recognizable, but a slight dilatation of a biliary duct can be appreciated (arrow in d)
Ultrasound is usually the first imaging method to discover ICC, although quite often it is not possible to differentiate it from other focal liver lesions, due to unspecific imaging findings [3, 23].
ICC shows an inhomogeneous hypoechoic appearance, with irregular margins (Fig. 42.3). After contrast media injection (CEUS), in mass-forming ICC, an arterial rim enhancement is typically seen (Fig. 42.4a) [24], although in the portal and late phases, ICC is typically hypoechoic in comparison to perilesional parenchyma (Figs. 42.3 and 42.4b) [25–27].
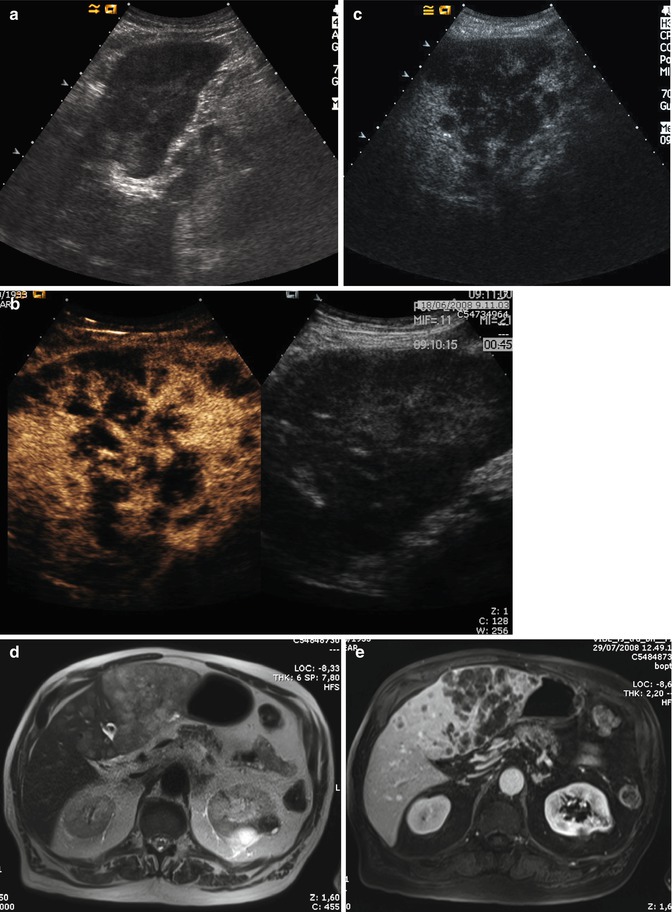
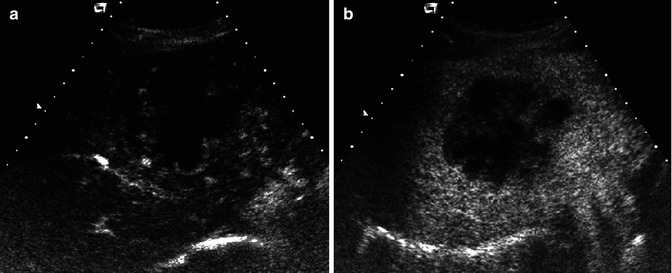

Fig. 42.3
(a–e) Multinodular ICC. Male, 74-years-old. (a) US, sagittal view on the left lobe. Inhomogeneous iso-/hypoechoic lesions can be appreciated; (b, c). CEUS: lesions appear hypovascular during dynamic acquisition (b), with marked hypoechoic appearance in late phase (c); (d, e) MR: lesions appear hyperintense on T2w (d), with prevalent location in the left lobe and marked hypovascular during portal phase after GBCA administration (e)

Fig. 42.4
(a, b). Mass-forming ICC. CEUS. Male, 65-years-old. A large mass in the right lobe can be appreciated, which shows a peripheral enhancement during the early dynamic acquisition (a) and a marked hypointensity during the late phase (b)
Small lesions (<3 cm) frequently show a homogeneous arterial enhancement [28], due to poor presence of fibrosis and desmoplastic reaction [29]. With CEUS, thanks to real-time analysis of contrast arrival, the early arterial enhancement is more easily appreciated than with CT, where it can be less evident or missed during the conventional time of acquisition of late arterial phase (25”–30”) [30, 31] (Fig. 42.5).
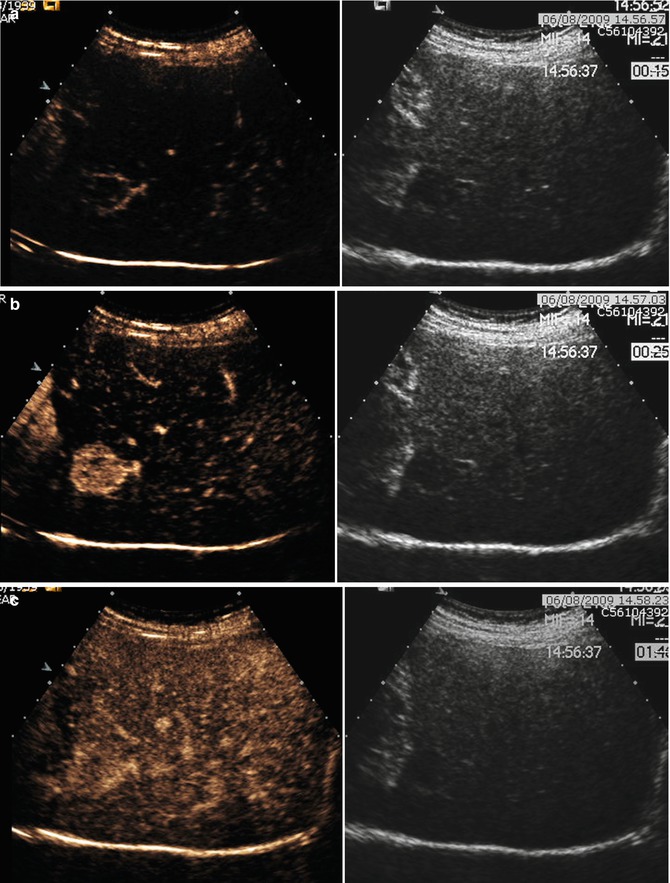
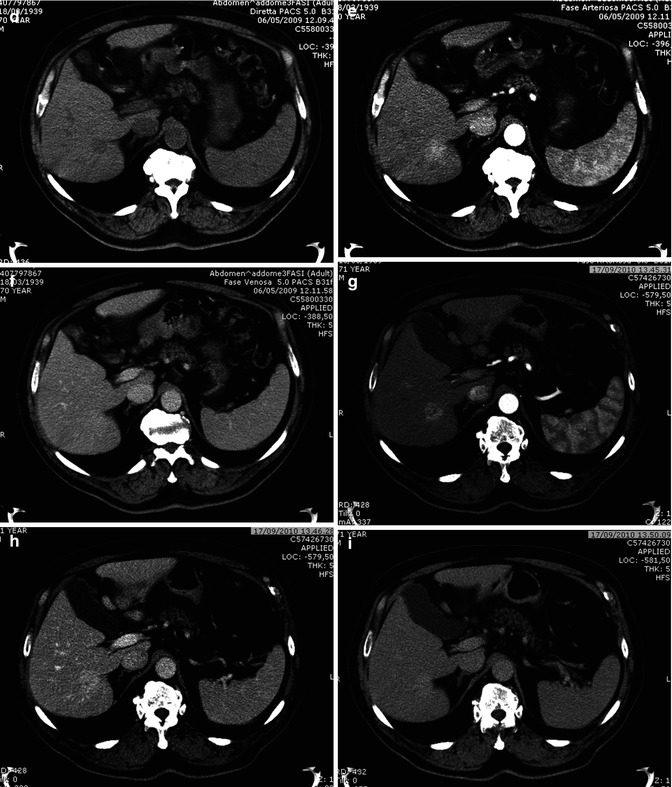


Fig. 42.5
(a–i). Small hypervascular ICC. CEUS vs TC. Male, 71-years-old. (a–c) CEUS. A small early enhancing hypervascular mass (a) can be appreciated in the right lobe, which does not show washout in portal and late phase (b, c). (d–f) CT. A small hypodense lesion is seen is the right lobe (d), which shows a slight hyperenhancement during the arterial phase (e), without washout in the portal phase (f). Patient refused biopsy and thus was sent for follow-up. (g–i) CT, 16 months later. The lesion is increased in diameter, with inhomogeneous arterial enhancement (g), which persists during portal and distribution phase (h, i). Pathological specimen revealed moderately differentiated ICC
42.2.1.1 Computed Tomography
At unenhanced scan, ICC appears as a hypodense lesion with irregular margins. After the intravenous administration of contrast material, most CHCs show a peripheral continuous rim enhancement during the arterial and portal-venous phases, while the center of the lesion remains hypoattenuated and shows enhancement during the delayed phase, findings that reflect their desmoplastic composition [32] (Fig. 42.6a–d).
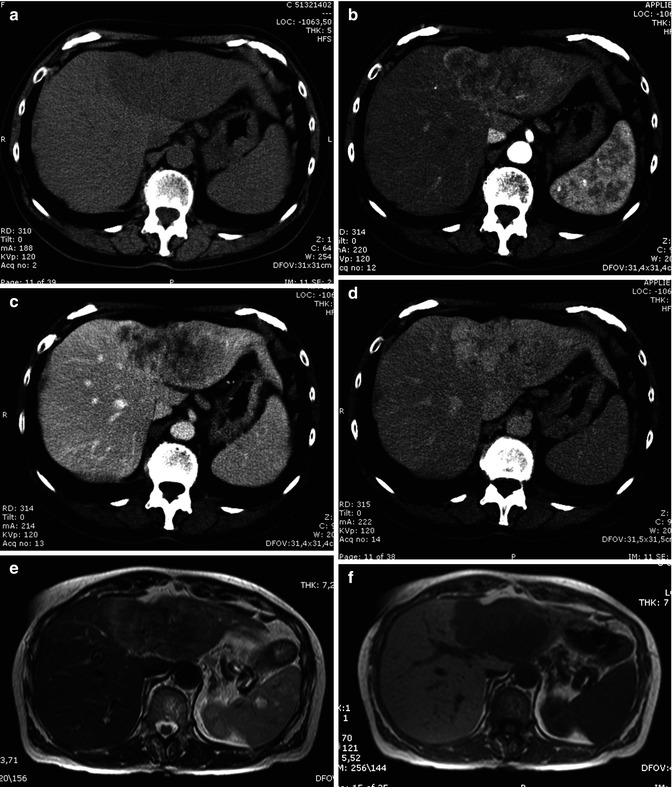
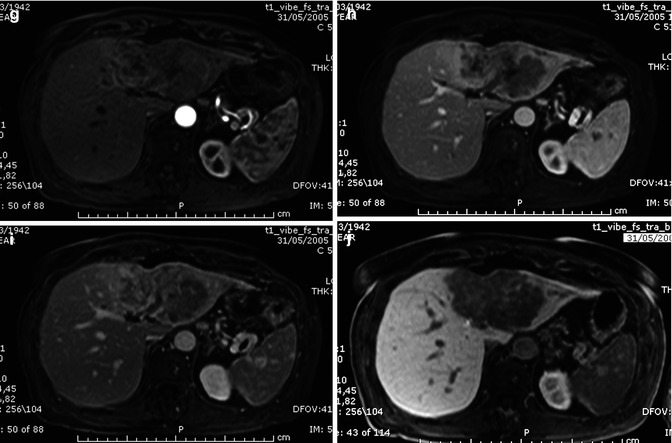


Fig. 42.6
(a–j) Large ICC. Female, 67-years-old. (a–d) CT. On precontrast acquisition, a large hypodense mass in the left lobe (a), which shows an arterial rim enhancement (b), with progressive central enhancement in the portal (c) and distribution phase (d). (e–l) MRI. On T2w (e), the lesion appears slightly hyperintense at the periphery while in the center is hypointense. On T1w (f), the lesion is homogeneously hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows a dynamic enhancement similar to CT, with an arterial rim enhancement (g) and with progressive central enhancement in the portal (h) and distribution phase (i). During hepatobiliary phase (j), 2 h after LSCA administration, the lesion appears hypointense, with slight enhancement in the central part, due to pooling in the fibrotic component
Less frequently, small lesions (<3 cm) show a more intense arterial enhancement, which involves more than 50 % of the lesion, with inhomogeneous or lack of washout in the portal phase [33], which reflects a lower content of fibrosis and necrosis and a higher content of neoplastic cells (Fig. 42.5d–i). This tumor has a better prognosis than conventional one [34], with a higher disease-free overall survival [35].
42.2.1.2 Magnetic Resonance
CHC is usually hypointense on T1-weighted images and shows a variable hyperintensity on T2-weighted images, depending on the amount of fibrous tissue, coagulative necrosis, and mucin in the core of the mass. The more the fibrotic changes, the lower the signal on T2-weighted images, although the amount of coagulative necrosis can greatly influence the signal intensity, either in terms of increasing it or decreasing it (Fig. 42.6) [36]. With gadolinium administration, it is possible to differentiate the fibrotic component from coagulative necrosis, as contrast uptake in the late phase images is only seen in the fibrotic component (Figs. 42.6 and 42.7) [21, 37]. Moreover, hypointensity on T2-weighted images is a characteristic of ICC but can be found also in some liver metastases [38].
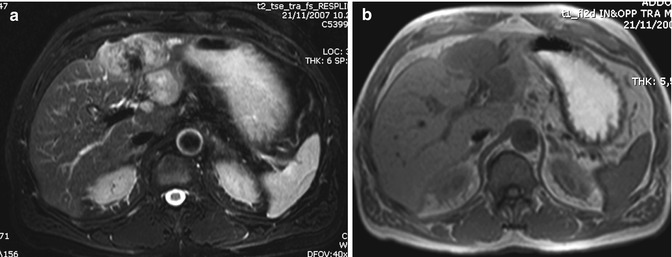
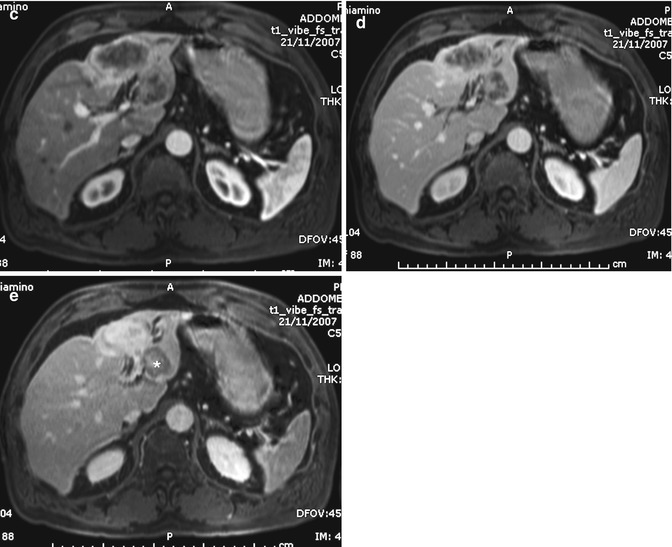


Fig. 42.7
(a–e) Large ICC. Male, 60-years-old. MRI. A large inhomogeneous mass in the left lobe is hyperintense on T2-weighted (a) and hypointense on T1-weighted images (b). During dynamic acquisition after GBCA administration, the lesion shows arterial rim enhancement (c), with progressive central enhancement during portal (d) and distribution phase (e). In the distribution phase, part of the lesion shows a target sign, due to pooling in the desmoplastic reaction at the core of the lesion, while the rest does not show central pooling, probably due to coagulative necrosis in the center of the lesion
After gadolinium administration, ICC shows a typical arterial peripheral enhancement, with progressive central enhancement in the late phase (2–5 min), according to the microscopic composition of the tumor (Figs. 42.6g–i and 42.7c–e) [21, 29]. However, this enhancement pattern is not typical only for ICC, as some hepatic metastases, especially from colorectal carcinoma, can show the same pattern [39].
Lack of rim enhancement or of central pooling has also been described, although is less frequent. Sometimes, a late rim enhancement around the tumor can be appreciated, due to a lower vascular supply in congested and dilated sinusoids [21].
A small cholangiocarcinoma may manifest as a hypervascular tumor; it may be seen in a well-differentiated tumor with abundant tumor vasculature in the fibrotic stroma in the absence of remarkable necrosis or displaying abundant tumor cells and a small degree of fibrosis. Prolonged enhancement may be attributed to the presence of the vascular fibrotic stroma (Fig. 42.8) [18, 33, 40–42].
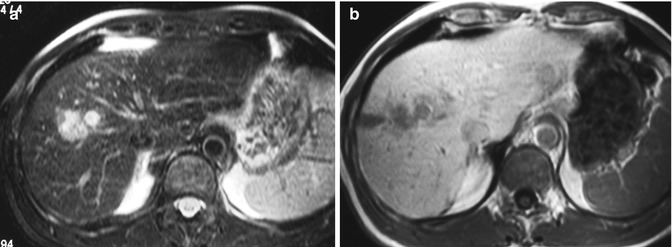
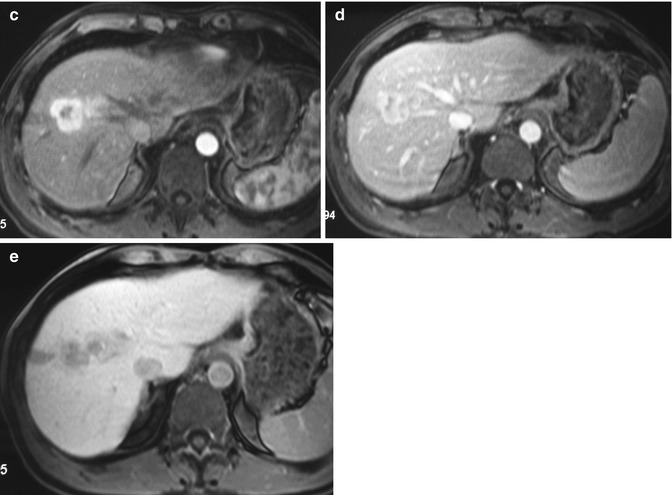


Fig. 42.8
(a–e) Hypervascular ICC. Male, 54-years-old. MRI. A lesion in the right lobe with a satellite nodule can be appreciated. On T2-weighted sequence (a), the lesion is hyperintense. On T1-weighted sequence (b), the lesion is hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows a large peripheral arterial enhancement (c), without significant washout during the portal phase (d). During hepatobiliary phase (e), 2 h after LSCA administration, the lesion appears hypointense
42.2.1.3 MR Liver-Specific Contrast Media
The use of liver-specific contrast media (LSCM) (Gd-BOPTA, MultiHance, Bracco, Milano, Italy; Gd-EOB-DTPA, Primovist, Bayer, Berlin, Germany) allows to obtain both morphological and functional information, thus permitting greater confidence in the imaging of focal liver lesions [43]. In case of ICC, LSCM allow a better characterization of the mass and a higher sensitivity in detecting satellite nodules and intrahepatic metastases [44], which is a negative prognostic factor.
As a rule, with these contrast agents, during dynamic imaging, ICC behaves in the same manner as with conventional extracellular gadolinium-based contrast agents (GBCA), while during hepatobiliary phase (2 h after Gd-BOPTA; 20 min after Gd-EBO-DTPA), the mass appears hypointense, as neoplastic cells are not able to uptake this contrast agent, due to the absence of hepatocytes (Figs. 42.6j and 42.8e).
While during the arterial and portal phase imaging findings do not differ significantly among the two agents (Gd-BOPTA and Gd-EOB-DTPA), during the late and hepatobiliary phase, a significant difference can be appreciated: with Gd-EOB-DTPA, an hypointense appearance of ICC can be observed already in the late phase (Fig. 42.9), because an uptake of Gd-EOB-DTPA by normal parenchyma can be detected even at 1–2 min after the contrast injection [44]. This effect has been called pseudo-washout, and it has been observed also in capillary hemangiomas [45]. With Gd-BOPTA, an uptake of contrast agent can be observed in the core of the lesion, due to the pooling of CA in the desmoplastic component of the mass (Fig. 42.10) [46]. Thus, the lesion can show only a slight hypointensity in comparison to the normal parenchyma, also due to a lesser hepatic uptake of this contrast agent in comparison to Gb-EOB-DTPA [47]. With Gd-BOPTA, quite often a hypointense peripheral rim in the hepatobiliary phase can be observed, due to the vital neoplastic component of the tumor, unable to uptake the contrast agent, which contrasts with the central hyperintense core due to pooling (target sign: Fig. 42.10e). Rarely, the central core is composed only by coagulative necrosis, unable to pool the contrast agent; thus, the lesion has a homogeneous hypointense appearance [48].
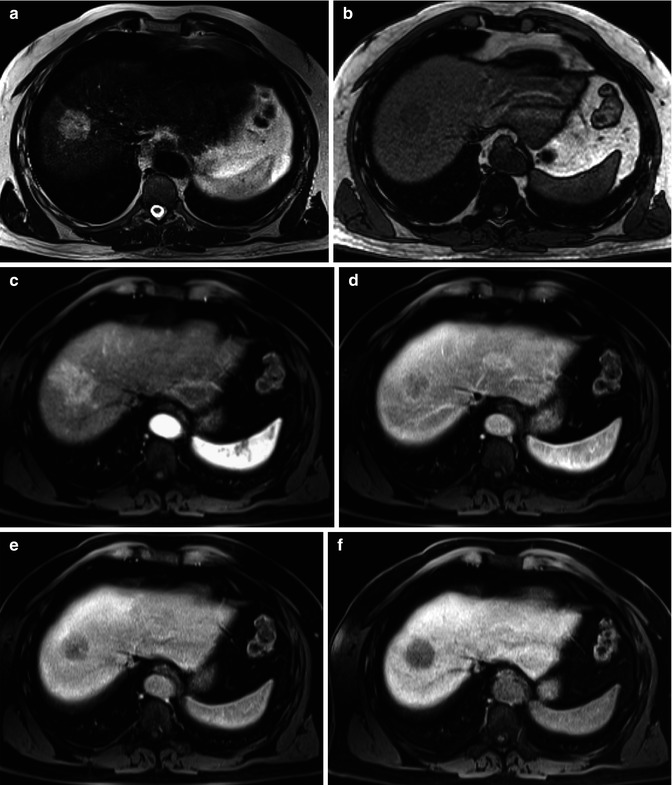
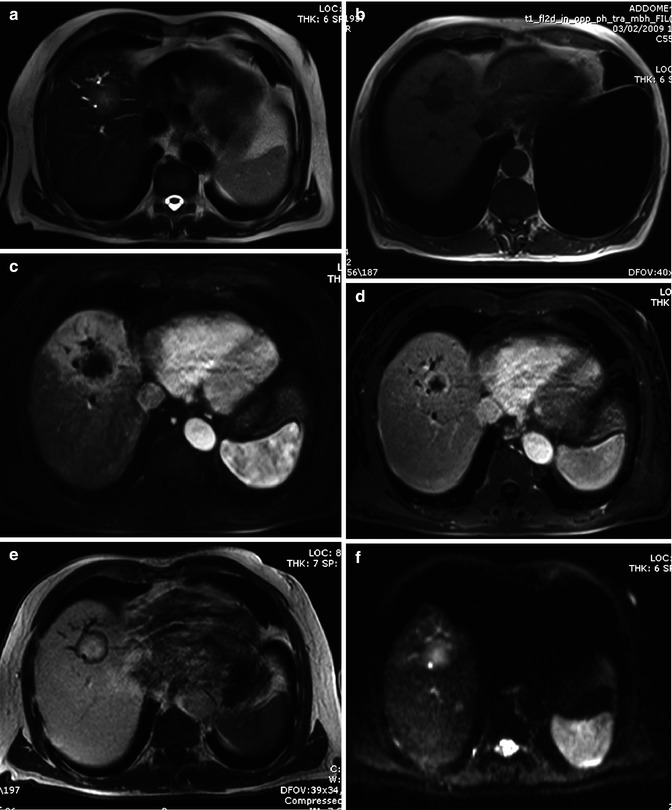
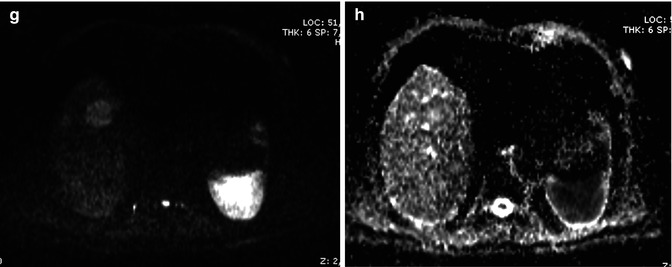

Fig. 42.9
(a–f) Hypervascular ICC. Male, 52-years-old. MRI. A round lesion in the right lobe can be appreciated. On T2-weighted (a), the lesion is hyperintense. On T1-weighted (b), the lesion is hypointense. During dynamic acquisition after LSCA administration (Gd-EOB-DTPA), the lesion shows homogeneous arterial enhancement (c), with washout during the portal (d) and distribution phase (e). During hepatobiliary phase (f), 20 min after LSCA administration, the lesion appears markedly hypointense. No target sign can be appreciated (Courtesy of Luigi Grazioli, MD. Ospedali Civili Brescia, Italy)


Fig. 42.10
(a–h). Mass-forming ICC. Male, 72-years-old. MRI. A round lesion in the right lobe can be appreciated. On T2-weighted image (a), the lesion is hyperintense. Dilatation of biliary tree upstream the lesion is present. On T1-weighted sequence (b), the lesion is hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows arterial rim enhancement (c), with partial centripetal enhancement during the portal phase (d). During hepatobiliary phase (e), 2 h after LSCA administration, the lesion shows marked pooling of contrast media in the fibrotic core with hypointense appearance in the peripheral cellular component, thus giving a target appearance. DWI, b 50 (f), b 800 (g), ADC map (h). At high b value (g), the lesion shows a target appearance, with a central hypointense area and a peripheral hyperintense rim, which reflects the different compositions of the lesion, with a central fibrous core and a peripheral rim of tumoral cells
42.2.1.4 Diffusion-Weighted Imaging
MR diffusion-weighted imaging (DWI) is a widely accepted technique in neuroradiology for detecting early ischemia in cerebrovascular accidents and characterization of brain tumors and intracranial infections but has ceased to be used only in brain applications for several years. DWI relies on measuring diffusion of water molecules in the tissue by MRI, related to their molecular mobility which reflects tissue properties such as the size of the extracellular space, viscosity, and cellularity.
Application of DWI has shown a sensitivity and accuracy higher than with the T2 TSE techniques in the detection of focal liver lesions [49], especially for small (≤10 mm) metastatic lesions [50]. Similar results have been found with ICC, which show higher contrast between the lesion and normal parenchyma with DWI images than with conventional T2-weighted images (Fig. 42.11) [51]. In comparison with conventional gadolinium-enhanced MRI, DWI has shown similar performance in the detection of hepatic metastases, with a significant lower specificity [52] but lower than with liver-specific gadolinium contrast agents [53, 54].
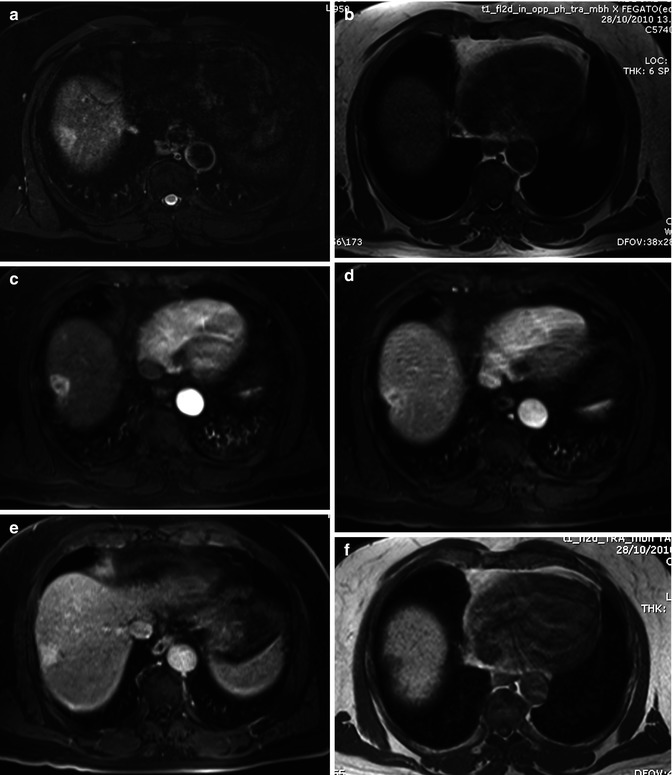
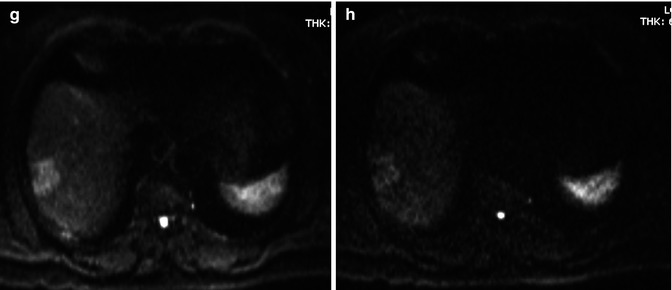


Fig. 42.11
(a–h) Moderately differentiated ICC in cirrhotic liver. Male, 60-years-old, with HBV cirrhosis. MRI. A peripheral lesion with capsular retraction can be appreciated in the right lobe. On T2-weighted sequence (a), the lesion is hyperintense. On T1-weighted image (b), the lesion is hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows homogeneous arterial enhancement (c), without significant washout during the portal phase (d), and progressive central enhancement during the distribution phase (e). During hepatobiliary phase (f), 2 h after LSCA administration, the lesion appears hypointense. DWI, b 50 (g) and b 600 (h). The lesion shows increase of signal intensity at high b value
As regards characterization, overlap in ADC values is reported between benign and malignant solid hepatic lesions [55]; however, ICC shows the lower ADC value between all the malignant lesions, thus making the differential diagnosis with benign lesion feasible [56]. In small ICC, a target appearance on DWI (a central hypointense area with a peripheral hyperintense rim) was the most reliable imaging feature for distinguishing small mass-forming ICC from small HCC (Fig. 42.10f–h).
42.2.1.5 PET-CT
Thanks to accumulation of FDG in the neoplastic cells, PET has gained an important role in the management of oncologic patients [57]. Hybrid technique (PET-CT), which fuses metabolic PET images to detailed anatomical CT images, overcomes the limits of PET in the anatomical assessment of metabolic alterations.
FDG uptake is linked to several factors: density of tumoral cells, tumoral grading, and percentage of vital cells [58]. FDG is taken up by cancer cells via facilitative glucose transporters (Gluts) [59]. Glut1 is not expressed in normal bile ducts but has been described to be strongly expressed in CHC [60, 61]. At PET, ICC shows intense FDG uptake, with often a characteristic central photopenia, corresponding to the central core of fibrotic tissue and desmoplastic reaction provoked by the neoplastic cells [62].
As a general rule, sensitivity of PET in detecting CHC is lower than CT but with ICC sensitivity is quite high, up to 90–95 % [63–68]. However, the role of PET in the assessment of ICC is more linked to staging and follow-up.
In the staging, FDG-PET can detect regional lymph node metastases undetected by CT, changing treatment plans in a significant number of cases, even though the sensitivity of PET was only 50 % [67]. Kato et al. reported 100 % specificity for regional nodal involvement on FDG-PET [69].
The most important role of PET in the staging of CCC is the detection of distant metastases. PET is able to change treatment plans in about 17–30 % of cases, especially due to the detection of distant metastases undetected at CT [63, 64, 66–68, 70], thus reducing useless laparotomy.
With regard to therapeutic monitoring and prognostic information with PET, very few studies are still available, and many of these are almost anecdotical.
PET is capable to identify residual tumor after percutaneous treatment before other imaging techniques [71], although these studies have been performed in other solid malignant hepatic lesions than ICC. However, very few studies have investigated the role of PET in monitoring therapeutic response, such as in cases of photodynamic therapy for unresectable hilar cholangiocarcinoma. In one study with ten patients, SUV-associated parameters did not show a significant change after treatment, while the estimated vital tumor volume even increased [72].
42.2.2 ICC in the Cirrhotic Liver
The most pathological clue of ICC arising in cirrhotic livers is that these lesions are more often hyperenhancing in comparison to ICC arising in noncirrhotic livers (Fig. 42.11). This enhancement behavior is due to an increase of the density of arterioles and capillaries [73]. This makes differential diagnosis with HCC difficult with the different imaging techniques [74–76], especially with small ICC (<2–3 cm), which typically shows a homogeneous enhancement without washout [33, 41, 42], similar to early HCC [77]. Typically, ICC in cirrhotic liver never shows washout in late phase imaging (Fig. 42.12) [42].
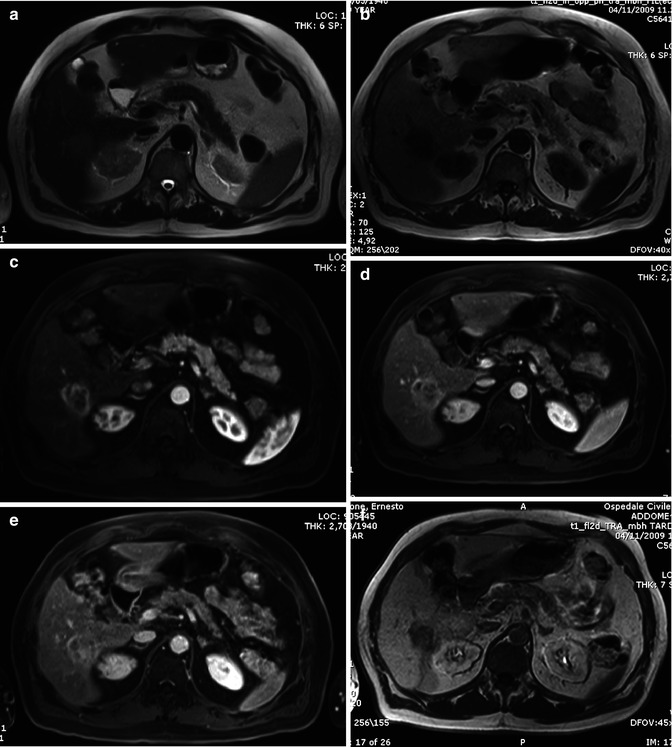

Fig. 42.12
(a–f) Low differentiated ICC in cirrhotic liver. Male, 71-years-old, with HCV cirrhosis. MRI. A peripheral lesion can be appreciated in the right lobe. On T2-weighted sequence (a), the lesion is hyperintense. On T1-weighted sequence (b), the lesion is hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows a thick peripheral arterial enhancement (c), with progressive central enhancement during the portal (d) and distribution phase (e). During hepatobiliary phase (f), 2 h after LSCA administration, the lesion appears hypointense
42.2.3 Differential Diagnosis
HCC : mass-forming ICC usually appears as a lesion with lobulated or irregular margins, noncapsulate, in comparison to the frequent round shape of HCC. However, imaging appearance of HCC can greatly vary as it may show lobulated margins in case of confluent nodules of multifocal HCC, extracapsular extension, desmoplastic reaction (scirrhous HCC), or infiltrative pattern of growth.
Biliary tree dilatation and capsular retraction are more typical for mass-forming ICC (Fig. 42.1a, b), while pseudocapsule is more typical for HCC (Fig. 42.13). Differently to ICC, HCC tends to invade vessels [4, 17]. Rarely, HCC can invade biliary vessels, thus simulating a polypoid ICC.
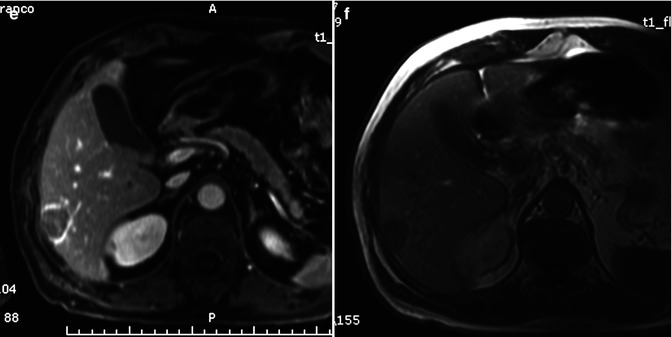


Fig. 42.13
(a–f) Moderately differentiated HCC in chronic hepatitis. Male, 68-years-old, with chronic hepatitis. MRI. A round peripheral lesion with sharp margins can be appreciated in the right lobe. On T2-weighted image (a), the lesion is hypointense with a perilesional hyperintense rim. On T1-weighted image (b), the lesion is isointense. The perilesional rim is hypointense. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows a homogeneous hyperenhancement in the arterial phase (c), with marked washout in the portal (d) and distribution phase (e). During the latter phases, a progressive enhancement of the perilesional rim can be appreciated (pseudocapsule). During hepatobiliary phase (f), 2 h after LSCA administration, the lesion appears hypointense
In HCC, contrast enhancement shows a typical pattern, with wash in during arterial phase and washout during portal and late phase (Fig. 42.13c–e) [78]; however, about 10–20 % of HCCs are hypovascular, thus giving some difficulties in differential diagnosis [79]. Finally, some peculiar form of HCC can mimic ICC during dynamic imaging: sclerosing HCC and fibrolamellar HCC contain significant fibrous tissue, thus showing an enhancement pattern which can simulate an ICC [80, 81].
The most difficult differential diagnosis arises with small homogeneous hypervascular ICC with or without washout, which can be difficult to differentiate from small HCC, and with inhomogeneously hypervascular ICC, which can give problems of differential diagnosis with large inhomogeneous HCC. In this latter case, in HCC, the mass volume of hyperenhancement is larger (>75 %) than ICC, which usually shows a thick peripheral enhancement, with sparing of central core [82].
Evaluation of time-intensity curve with CEUS can be helpful in differentiating focal liver lesions [83]: usually, ICC shows a wash in with a lower peak both at the center and at the periphery of the lesion in comparison to HCC, although the time to peak is almost the same, while the washout in ICC is much more evident at the periphery of the lesion [28]. According to these authors, with this quantitative technique, it is possible to differentiate ICC from HCC with an accuracy similar to CT or MRI [28], although in clinical practice it is not widely applied.
With ICC, washout tends to be earlier than with HCC, but there is no consensus on the diagnostic value of this parameter [28, 76, 84].
With MRI, signal inhomogeneity can be appreciated both in HCC and ICC, especially on T2-weighted images. On ICC, both hypointensity and hyperintensity can be appreciated due to fibrosis and coagulative necrosis, respectively. On HCC, hemorrhage and colliquative necrosis are more frequent, thus giving hyperintensity on both T1-weighted and T2-weighted images.
With LSCAs, HCC is usually hypointense during the hepatobiliary phase, due to the lack of contrast uptake in the neoplastic cells [43, 48], while ICC shows quite often the target sign (Fig. 42.10e), due to the pooling of contrast agent in the desmoplastic component of the core of the lesion, especially with Gd-BOPTA. However, some HCCs, especially well-differentiated and scirrhous ones, can show late hyperintensity within the lesion, due to residual hepatobiliary activity or for the presence of fibrous stroma in case of scirrhous HCC (Fig. 42.14) [43, 48, 78, 85, 86].
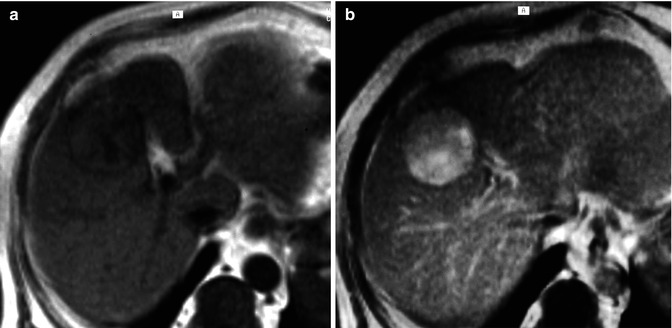

Fig. 42.14
(a, b) Moderately differentiated HCC. MRI, T1-weighted images before (a) and 2 h after (b) LSCA (Gd-BOPTA) administration. The lesion is isointense in the unenhanced images but shows a marked enhancement during the hepatobiliary phase
Hepatocholangiocarcinoma (HCHC) is a rare form of tumor (<1 % of hepatic tumors) [87]. According to some authors, these hepatic tumors could take origin from hepatic progenitor cells (HPC or tumor-initiating stem-like cells) [88–91], which are capable to differentiate in both hepatic and cholangiocellular tumors [92–94], with clinical and epidemiologic findings similar to that of HCC and ICC [95–97].
According to WHO classification, HCHC can be subdivided in classical type and subtypes with stem cell features, according to morphological and immunohistochemical features [98].
Imaging findings are related to the most represented cellular line, usually with HCC-type cells at the periphery of the lesion and ICC-type cells at the center, which show the corresponding imaging features (wash in and washout for HCC type and progressive enhancement for ICC type), at both CT and MRI [102–104].
During the hepatobiliary phase after MR LSCM, target sign is usually absent in HCC-prevalent type, while can be observed in ICC-prevalent-type lesions in about half of the cases [104].
Hypointensity on T2-weighted images, central fibrosis and coagulative necrosis, rim enhancement, and target sign can be present on both lesions (Fig. 42.15) [39, 105].
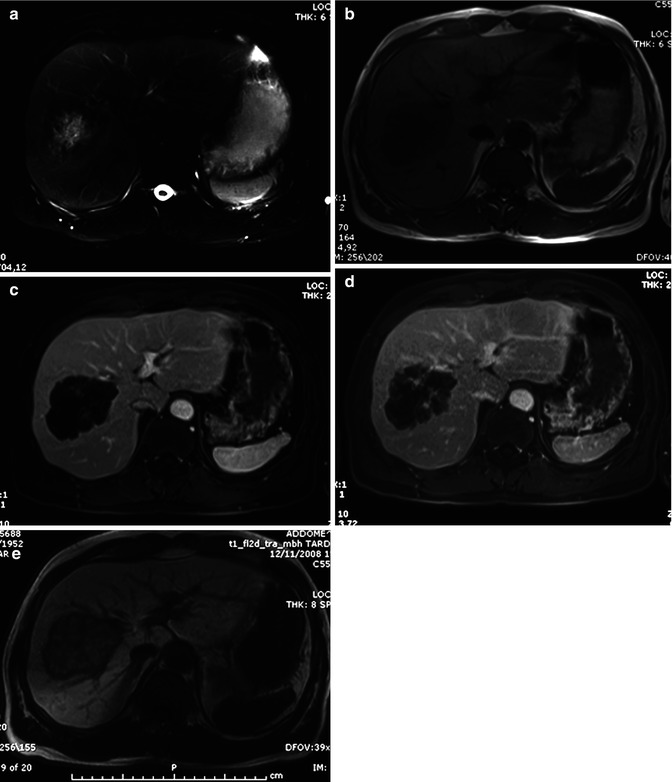

Fig. 42.15
(a–e) Metastases from colon carcinoma. Male, 57-years-old, with previous history of colon carcinoma. MR. A round lesion with lobulated margins can be appreciated in the right lobe. On T2-weighted image (a), the lesion shows a hyperintense core with a peripheral hypointense rim. On T1-weighted image (b), the lesion is hypointense, more evident in the central core. During dynamic acquisition after LSCA administration (Gd-BOPTA), the lesion shows a slight peripheral enhancement in the arterial (c) and portal (d) phase. During the hepatobiliary phase (e), 2 h after LSCA administration, the lesion shows a target appearance, with a central hyperintense pooling and a peripheral hypointense rim
Even contrast media administration is not helpful in differentiating ICC from hepatic metastases [39], also after liver-specific administration: the target sign during hepatobiliary phase can be observed also in some hepatic metastases [105], due to the presence of a central hypovascular core, where contrast media can be pooled. In the absence of a positive history of previous tumor, differential diagnosis can be difficult, although irregular margins, the absence of colliquative necrosis, and accessory findings (dilatation of biliary tree upstream the lesion (Fig. 42.1a), lobar or segmental hepatic atrophy (Fig. 42.1b), vascular encasement, and capsular retraction (Fig. 42.1c)) can be very helpful in the diagnosis of ICC [40, 105].
42.3 Malignant Vascular Tumors
In adults, primary malignant tumors of the liver arising from the vascular elements of mesenchymal tissue are uncommon and include angiosarcoma, epithelioid hemangioendothelioma, and Kaposi sarcoma.
42.3.1 Angiosarcoma
Primary hepatic angiosarcoma is a rare malignancy, accounting for 0.1–2 % of all malignant primary liver tumors [106–109], but it is the most common primary mesenchymal tumor of the liver, occurring more frequently than fibrosarcoma, malignant fibrohistiocytoma, and leiomyosarcoma. Angiosarcoma usually presents in late adulthood with median age at diagnosis of 50–59 years and affects male at a rate of 4:1 [108, 109].
In 7–10 % of cases, it has been associated with environmental and occupational exposure to carcinogens, such as vinyl chloride, thorium dioxide, arsenic, and radiation [110, 111]. It has been described in association with systemic diseases, such as hemochromatosis and von Recklinghausen disease [112, 113]. Nevertheless, causes of most of this tumor are unrelated to these risk factors and remain unknown.
Primary hepatic angiosarcomas progress rapidly, with a median survival of only 6 months without any treatment, and a majority of patients die within a year after diagnosis [109, 114].
The diagnosis of hepatic angiosarcoma is challenging, because patients usually present with nonspecific symptoms and laboratory test abnormalities. Abdominal pain, weakness, fatigue, and weight loss, with elevation of y-glutamyl transpeptidase and alkaline phosphatase, are frequent clinical features. Anemia (especially microangiopathic hemolytic) and thrombocytopenia are frequently associated [109, 114, 115].
Hepatomegaly and hemorrhagic ascites are also seen. In some cases, spontaneous hemoperitoneum is the first presentation of the tumor, secondary to the rupture of tumors arising in subcapsular locations of the liver.
Pathological examination has been proven to be the best approach to make diagnosis of primary hepatic angiosarcoma. Although some reports of potentially lethal complications following image-guided percutaneous biopsy are described [116], recent series reported that percutaneous biopsies in some series yielded diagnostic specimens without substantial bleeding [117].
At pathological evaluation, angiosarcoma appears, macroscopically, as a necrotic and hemorrhagic lesion with four types of growth patterns: multiple nodules, large dominant mass, mixed patterns of a dominant mass with nodules, and diffusely infiltrating micronodular tumor [118, 119]. Microscopically, angiosarcoma is characterized by the proliferation of malignant endothelial spindle cells arranged in poorly organized vascular channels or cavernous spaces, forming solid nodules or masses, both infiltrating surrounding hepatic tissue in a destructive fashion [118, 119]. Solid portions of the tumor contain fibrosis and hemosiderin deposition [118, 119]. Immunohistochemically, the tumor is positive for CD31, CD34, and vimentin, a mesenchymal marker, and is negative for HAS and GPC-3, which excludes the possibility of hepatocyte origin.
42.3.1.1 Imaging Findings
Histopathologic diagnosis should be correlated with radiological findings to avoid error in diagnosis. Imaging features of angiosarcoma on imaging reflect the pleomorphic architecture observed on histopathology.
Ultrasound
Ultrasound features of neoplastic nodules or mass vary according to the degree of necrosis and hemorrhage and result of hyperechoic and hypoechoic areas, with fluid/fluid level.
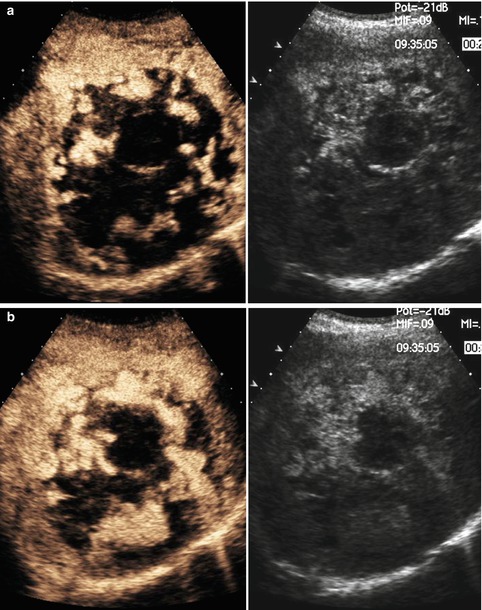
Recently, some studies show a diagnostic value of CEUS for diagnosis of primary hepatic angiosarcoma, characterized by a peripheral irregular enhancement with a remarkable unenhancement of the central part of the tumor during the arterial and portal phase (Fig. 42.16a, b), which shows complete washout in the late phase [120].
Get Clinical Tree app for offline access


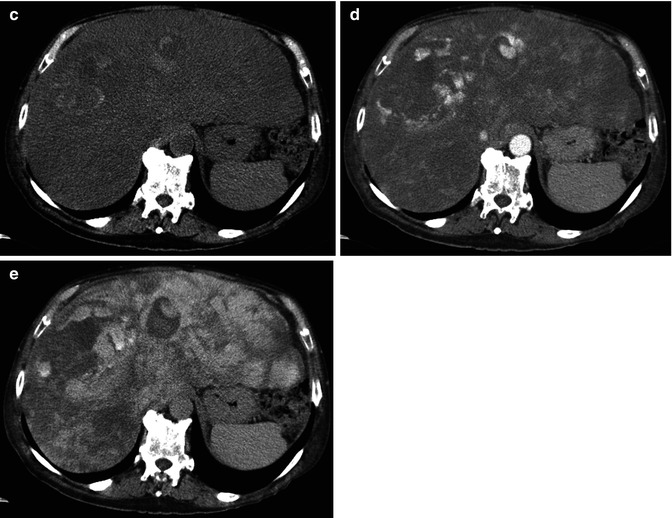
Fig. 42.16
(a–e) Angiosarcoma. A male, 73-years-old, with previous exposure to vinyl chloride, was admitted at Emergency Department for abdominal pain. At US, a large mass with diffuse alterations of echotexture was appreciated in the right lobe (not shown). (a, b) CEUS shows prevalent peripheral globular enhancement of the lesion. (c–e) CT. At unenhanced phase (c




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree