Abstract
Focal FDG avidity within the pancreas is suspicious for a focal pancreatic malignancy. However, there are many inflammatory causes of FDG avidity such as pancreatitis (such as secondary to pancreatic duct obstruction, IgG4 disease, or vincristine therapy), pancreatic stents, and postoperative changes. Pancreatic ductal dilatation may be the only visible manifestation of a focal pancreatic abnormality. The pancreatic ductal should be evaluated on every CT, and pancreatic duct dilatation should prompt evaluation for a cause.
Keywords
FDG, PET/CT, pancreas, pancreatic cancer, pancreatitis
The differential for focal pancreatic lesions depends on whether the lesion is solid or cystic. Solid pancreatic lesions include primary pancreatic adenocarcinoma, pancreatic neuroendocrine tumors (PNETs), lymphoma, and metastases. Cystic pancreatic lesions include true cysts, pseudocysts, and cystic neoplasms. Focal fluorodeoxyglucose (FDG) avidity within the pancreas is suspicious for focal pancreatic lesion. Obstruction of the pancreatic duct can result in pancreatic inflammation, such as pancreatitis, which causes longer segments of FDG avidity in the affected regions of the pancreas.
Solid Pancreatic Lesions
Pancreatic Adenocarcinoma
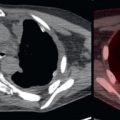
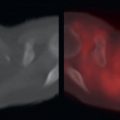
Primary pancreatic adenocarcinomas vary widely in their FDG avidity, ranging from markedly FDG avid to not appreciable on FDG positron emission tomography (PET). Do not allow mild or lack of FDG avidity in a primary pancreatic adenocarcinoma to suggest a benign lesion, because almost all pancreatic adenocarcinomas are highly aggressive, even if not apparent on FDG PET. The variability in FDG avidity makes detection of the primary pancreatic neoplasm unpredictable on FDG PET/computed tomography (CT), and contrast-enhanced CT or magnetic resonance (MR) imaging at a pancreatic contrast phase (approximately 45 seconds) remains the mainstay for imaging primary pancreatic malignancies and determining their resectability. Pancreatic adenocarcinomas are often hypoattenuating to normal pancreatic parenchyma on contrast-enhanced CT; however, a small percentage are isoattenuating, and FDG PET may provide value in visualizing the primary pancreatic adenocarcinoma in this small percentage of patients. Overall, the detection of the primary malignancy is usually not the primary purpose of FDG PET/CT, even if the primary malignancy is FDG avid in many patients ( Fig. 14.1 ).

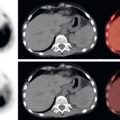
As with malignancies from many other organ systems, FDG PET/CT is far more useful for the detection of nodal and distant metastases from pancreatic adenocarcinoma than for detection of the primary pancreatic malignancy. FDG avidity within borderline-sized local lymph nodes may help to raise confidence in diagnosis of nodal metastases, and FDG-avid foci within the liver of a patient with pancreatic adenocarcinoma is highly suspicious for hepatic metastases ( Fig. 14.2 ). Peritoneal carcinomatosis may be visualized on CT, FDG PET, both (see Fig. 14.1 ), or neither. It is not unusual for peritoneal carcinomatosis to be detected during surgery which was occult on both CT and FDG PET.

Surgery is the only current potentially curable treatment modality for pancreatic adenocarcinoma; unfortunately, most patients present with unresectable disease. Systemic chemotherapy with or without radiation is used in most patients. Despite the method of initial therapy, most patients will have residual or recurrent malignancy within 2 years. Postsurgical changes may make detection of recurrent malignancy difficult on anatomic imaging, and FDG PET/CT has shown promise in detecting recurrent malignancy following therapy ( Fig. 14.3 ).

Pancreatic Neuroendocrine Tumors
Neuroendocrine tumors represent a minority of pancreatic neoplasm, maybe 2%. Neuroendocrine tumors may be nonfunctioning, without secretion of hormones, or functioning, which may present with specific endocrine syndromes. The most common functioning pancreatic neuroendocrine tumor is insulinoma, followed by gastrinomas. Neuroendocrine tumors often demonstrate arterial phase enhancement on CT or MR, in contrast to pancreatic adenocarcinomas, which are usually hypoattenuating to normal pancreatic parenchyma. PNETs vary widely in their extent of FDG avidity. FDG avidity often correlates with histologic grade of a PNET, with well-differentiated PNETs demonstrating little or no FDG avidity, whereas poorly differentiated PNETs may be markedly FDG avid. Remember that this is unlike pancreatic adenocarcinomas, which also vary widely in their extent of FDG avidity but are almost always high-grade malignancies. The usefulness of FDG PET/CT for detection of primary and metastatic PNETs will vary based on the extent of FDG avidity. Of note, well-differentiated PNETs with low FDG avidity may be substantially better visualized by somatostatin-based nuclear imaging, such as 18F-DOTA-octreotate (18F-DOTATATE) PET/CT or indium-111 octreotide single-photon emission computerized tomography (SPECT)/CT.
Pancreatic Lymphoma
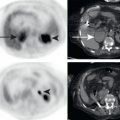
Lymphomatous involvement of the pancreas is relatively rare, found in less than 1% of all pancreatic neoplasms. Pancreatic lymphoma may be primary, originating in the pancreas, or secondary, originating elsewhere and secondarily involving the pancreas. Secondary pancreatic lymphoma is far more common than primary pancreatic lymphoma, and thus abnormalities elsewhere in the body, such as FDG-avid enlarged lymph nodes or splenomegaly, help to distinguish pancreatic lymphoma from other pancreatic neoplasms ( Fig. 14.4 ). Following the treatment of initially FDG-avid pancreatic lymphoma, the Lugano Criteria are used to determine treatment response. In brief, reduction of FDG avidity to less than liver background represents complete response to treatment, whereas residual FDG avidity greater than liver background is suspicious for residual active lymphoma.

Pancreatic Metastases
Metastases to the pancreases are also relatively rare, probably less than 2% of all pancreatic neoplasms. The primary malignancies that cause metastases to the pancreas are lung, breast, and melanoma. Metastatic disease is usually quite advanced before pancreatic metastases are recognized. They may be apparent as focal FDG avidity on FDG PET, masses on CT, or both.
Cystic Pancreatic Lesions
True Pancreatic Cysts
True pancreatic cysts are relatively rare and congenital. Multiple true pancreatic cysts may be seen in patients with autosomal dominant polycystic disease, von Hippel-Lindau syndrome, and cystic fibrosis. True pancreatic cysts demonstrate near-water attenuation on CT and are usually FDG photopenic.
Pancreatic Pseudocysts
Pancreatic pseudocysts are associated with acute or chronic pancreatitis. On CT, pseudocysts are usually near-water attenuation. Few data exist on the appearance of pseudocysts on FDG PET, although some have been noted to be FDG photopenic. If acutely inflamed, it would not be surprising to see FDG avidity.
Pancreatic Cystic Neoplasms
Cystic neoplasms are being increasingly diagnosed due to increased use and improvements in CT and MR imaging. Serous cystadenomas, mucinous cystic neoplasms, and intraductal papillary mucinous neoplasms (IPMNs) represent the vast majority of pancreatic cystic neoplasms. There are additional rare cystic neoplasms, and occasionally solid pancreatic lesions may undergo cystic degeneration. Due to overlap between the appearances of different pancreatic cystic neoplasm, it is often difficult to make a definitive diagnosis ( Fig. 14.5 ). However, an attempt should be made to differentiate categories of cystic malignancies. The diagnosis of a pseudocyst in a patient with a history of pancreatitis will prevent further workup of the lesion. Differentiating a pancreatic cystic neoplasm from a pancreatic adenocarcinoma is also crucial because the prognosis and treatment of pancreatic adenocarcinoma are dramatically different from those for pancreatic cystic neoplasms. Pancreatic cysts with solid components should be viewed as suspicious for more aggressive malignancy. After pseudocyst and cystic degeneration of a pancreatic adenocarcinoma are excluded, the management of a pancreatic cystic neoplasm can be quite variable. Management decisions, including imaging follow-up or surgical resection, may be based on the presence of symptoms, patient age, tumor size, and location.

Pancreatic Ductal Dilatation
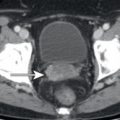
The presence of pancreatic ductal dilatation should be evaluated for on all CT scans which include the pancreas, even low-dose noncontrast CT examinations, which often accompany FDG PET. Pancreatic adenocarcinomas may be isoattenuating to the remainder of the pancreas on CT and are sometimes not FDG avid; thus dilatation of the pancreatic duct may be the only visible manifestation of a focal pancreatic abnormality on FDG PET/CT. If pancreatic ductal dilatation is identified, this should prompt evaluation for cause. The cause of pancreatic ductal dilatation may be chronic pancreatitis, which may be evident from pancreatic calcifications, pancreatic parenchymal atrophy, or inflammatory changes adjacent to the pancreas on the CT component of FDG PET/CT ( Fig. 14.6 ). If a cause is not evident on the FDG PET/CT images, further evaluation by MR or endoscopic evaluation may be warranted. In addition to obstructing stones, focal masses such as pancreatic adenocarcinoma ( Fig. 14.7 ) should be considered.