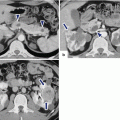
Fig. 11.1
Distribution of ductal adenocarcinoma in the pancreas
Practical Pearls
The location of the tumor in the pancreas determines its route of spread and the nodal groups involved.
A tumor in the anterior portion of the pancreatic head extends along the anterior pancreaticoduodenal artery and the proper hepatic artery.
A tumor in the posterior pancreatic head grows along the posterior pancreaticoduodenal vein toward the inferior portal vein surface.
A tumor in the cranial aspect of the pancreatic head near the gastrocolic trunk confluence may infiltrate the base of the transverse mesocolon.
A tumor in the uncinate process grows along the inferior pancreaticoduodenal arcade along the posterior surface of the superior mesenteric artery or into the jejunal mesentery.
Tumors in the pancreatic body and tail generally infiltrate along the splenic artery and the splenic vein to either the celiac axis or portal vein with potential for direct invasion of the spleen, peritoneum, stomach, colon, and left adrenal gland.
11.5 Histopathology
11.5.1 Macroscopic Appearance (Figs. 11.2 and 11.3)
White, firm, poorly defined pancreatic mass
Obstruction of the pancreatic duct, common bile duct, or both
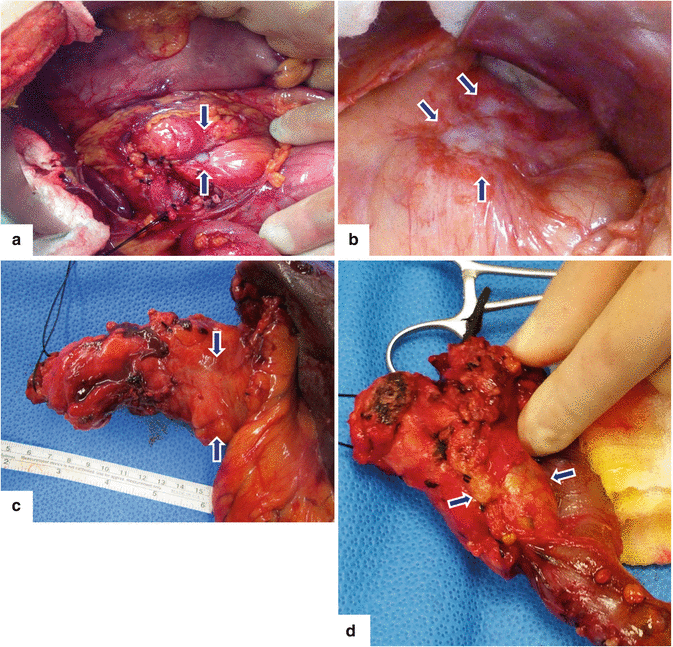
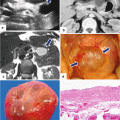
Fig. 11.2
Pancreatic ductal adenocarcinoma, macroscopic appearance. Intraoperative photographs show (a) a gray-white, infiltrative mass in the body of the pancreas producing umbilication of the peripancreatic tissues (arrows); (b) a white-tan, poorly defined mass involving the pancreatic body and tail (arrows). Photographs of gross specimens (c) shows a hard, infiltrative mass involving the tail of the pancreas (arrows) and (d) a lobulated mass involving the head and uncinate process of the pancreas (arrows)
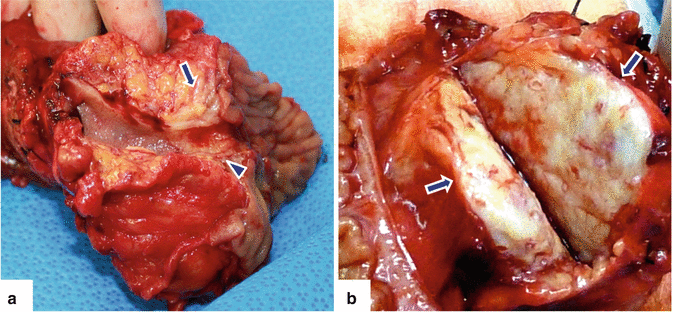
Fig. 11.3
Pancreatic ductal adenocarcinoma, macroscopic appearance. Photographs of two bivalved pancreaticoduodenectomy specimens show (a) a small yellow, invasive mass (arrow) extending into the common bile duct (arrowhead); (b) a firm, large, gray-white, poorly defined mass (arrows)
Practical Pearl
This tumor grows circumferentially. It begins by compressing, obstructing, infiltrating, and destroying the small ducts and then the large ductal structures.
11.5.2 Microscopic Appearance (Figs. 11.4–11.8)
Cell of origin: epithelium of the pancreatic ducts.
85–90 % of pancreatic cancers are adenocarcinomas.
Proliferation of small to large tubular glands lined by cuboidal to tall cells interspersed in an abundant desmoplastic stroma.
The degree of gland formation is proportional to the degree of differentiation, ranging from well-differentiated adenocarcinoma to infiltrating isolated cells or cells forming solid sheets in poorly differentiated adenocarcinomas.
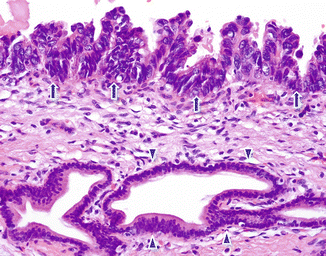
Fig. 11.4
Pancreatic ductal adenocarcinoma, microscopic appearance. Histological section shows the difference between a normal pancreatic ductal epithelium lined by with basally located oval nuclei cells (arrowheads) and malignant ductal cells composed of larger stratified nuclei with a papillary architecture (arrows) (H&E, 10×)
11.5.3 Histologic Grading
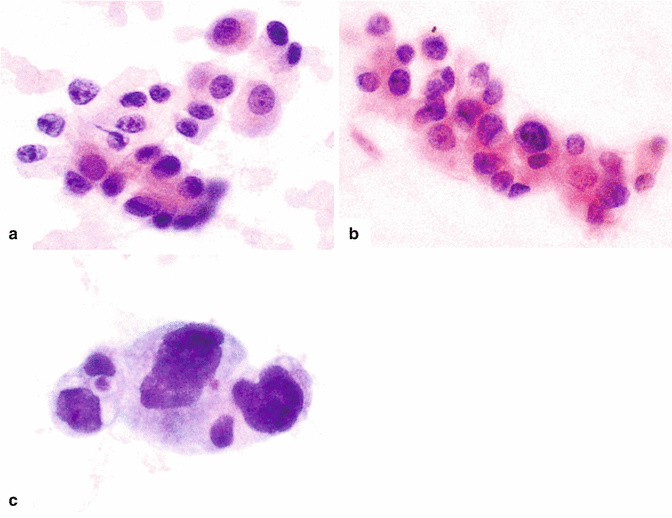
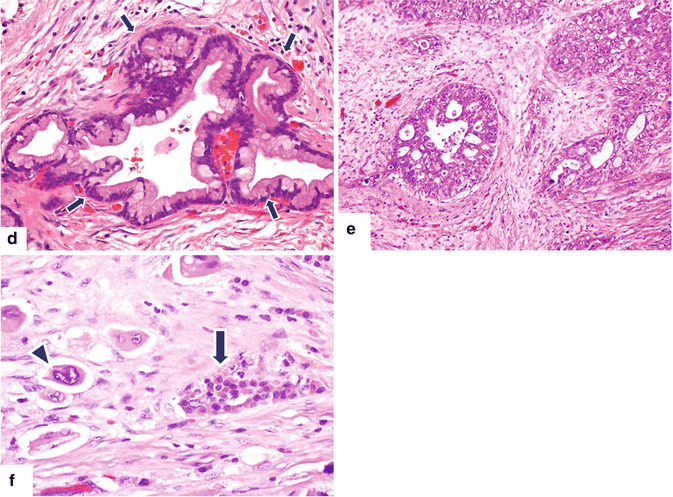
Fig. 11.5
Pancreatic ductal adenocarcinoma, cytologic appearance. Cytology smears (a–c) (Papanicolaou, 60×) demonstrate (a) cytology of a well-differentiated pancreatic adenocarcinoma. Note the mildly increased nuclear to cytoplasmic (N:C) ratio, the presence of nucleoli, and the architectural disarray. (b) Cytology of a moderately pancreatic adenocarcinoma. The nuclei are larger, more pleomorphic and tend to overlap. (c) Cytology of a poorly differentiated pancreatic adenocarcinoma. Note the markedly enlarged cells with high N:C ratio and irregular nuclear contours. Histological sections (d–f) demonstrate (d) histology of a well-differentiated pancreatic adenocarcinoma with desmoplastic reaction (arrows) (H&E, 10×), (e) histology of a moderately differentiated pancreatic adenocarcinoma with enlarged cells showing more pleomorphism and prominent desmoplastic stromal reaction (H&E, 20×), (f) histology of a poorly differentiated pancreatic adenocarcinoma with isolated large irregular cells (arrowhead) invading a residual islet of Langerhans (arrow) (H&E, 60×)
Grade 1 (well-differentiated)
Grade 2 (moderately differentiated)
Grade 3 (poorly differentiated)
This tumor is very invasive, growing into and along the pancreatic ducts.
Neural invasion is a very common finding (90 %), as well as the extension to peripancreatic fat tissues (Fig. 11.6).
Lymphatic and blood vessel invasion (Fig. 11.7) is also a common finding.
Invasion of the common bile duct, duodenal wall, and ampulla of Vater (Fig. 11.8) is frequently observed in carcinomas of the head of the pancreas.
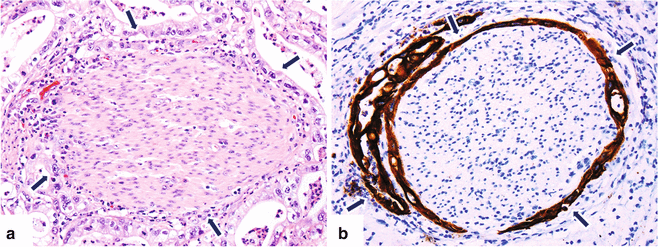
Fig. 11.6
Invasive pancreatic ductal adenocarcinoma, microscopic appearance. Histological sections (H&E, 40×) (a) show a nerve surrounded by adenocarcinoma cells with clear cytoplasm (arrows). (b) Keratin immunostain highlights the neoplastic cells surrounding the nerve (arrows) (immunoperoxidase, 40×)
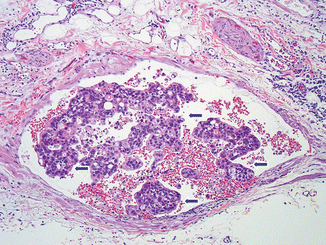
Fig. 11.7
Invasive pancreatic ductal adenocarcinoma, microscopic appearance. Histological sections demonstrates a blood vessel (vein) containing erythrocytes and tumor cells (arrows) (H&E, 10×)
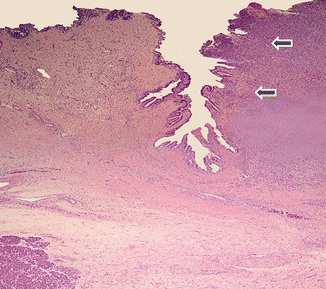
Fig. 11.8
Invasive pancreatic ductal adenocarcinoma, microscopic appearance. Histological section reveals moderately differentiated pancreatic adenocarcinoma infiltrating the ampulla (arrows) (H&E, composite)
11.5.4 Immunohistochemical Findings
Conventional pancreatic ductal adenocarcinoma show:
Focal mucin positivity by Alcian blue stain alone or combined with PAS stain.
Positive for pancytokeratin (CK) and epithelial membrane antigen (EMA).
Stain with CK 7, 8, 18, and 19.
CK20 is detected in less than 20 %.
Most of these tumors express:
MUC 1 (86 %), MUC4 and MUC5 (71 %), MUC6 (20 %), and MUC 2 (6 %)
CDX2 (14 %)
11.5.5 Other Histomorphologic Variants
Adenosquamous carcinoma (Fig. 11.9)
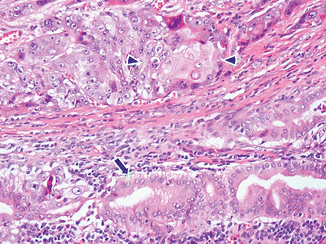
Fig. 11.9
Variant of pancreatic ductal adenocarcinoma, microscopic appearance. Histological section shows an adenosquamous-type pancreatic adenocarcinoma characterized by malignant glands (arrow) and the squamous carcinoma cells (arrowheads) (H&E, 40×)
Rare adenocarcinoma with areas of malignant squamous carcinoma (usually 30 % of any component).
Prognosis is worse than the usual type of ductal adenocarcinomas.
The areas of squamous differentiation are positive for p63 and CK5/6 immunostains.
Clear cell carcinoma (Fig. 11.10)
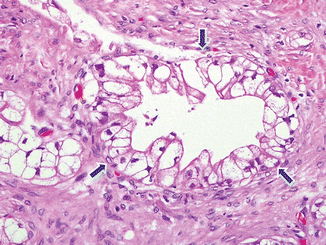
Fig. 11.10
Variant of pancreatic ductal adenocarcinoma, microscopic appearance. Histological section shows a clear cell-type pancreatic adenocarcinoma characterized by large pleomorphic cells with small pyknotic nuclei and abundant, clear cytoplasm (arrows) (H&E, 40×)
Variant with aggressive behavior
Adenocarcinoma composed of at least 75 % clear cells, not secondary to deposits of glycogen or mucin, and occasional condensed hyperchromatic nuclei
Negative for PAS and mucicarmine stains
Colloid adenocarcinoma (mucinous non-cystic) (Fig. 11.11)
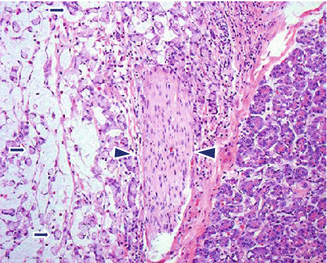
Fig. 11.11
Variant of pancreatic ductal adenocarcinoma, microscopic appearance. Histological section shows a colloid-type pancreatic adenocarcinoma, characterized by mucinous cells floating in abundant pools of mucin (arrows). Between the colloid adenocarcinoma and the normal pancreatic parenchyma is a nerve with perineural invasion (arrowheads) (H&E, 10×)
Adenocarcinoma cells suspended in large pools of extracellular mucin, representing more than 50 % of the tumor
Associated to intestinal type of IPMN
Positive for MUC2 and CDX2 immunostains (100%)
Better prognosis than usual adenocarcinoma
Foamy gland adenocarcinoma (Fig. 11.12)
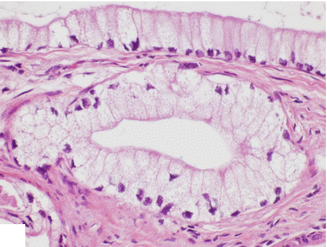
Fig. 11.12
Variant of pancreatic ductal adenocarcinoma, microscopic appearance. Histological section (H&E, 40×) shows a foamy gland variant pancreatic adenocarcinoma characterized by bland-looking glands with cells showing abundant foamy cytoplasm, basally located hyperchromatic, irregular nuclei, brush-like luminal cytoplasmic border and mild desmoplastic reaction (arrows) (H&E, 40×)
Adenocarcinoma composed of well-formed glands with cytologically bland cells with prominent foamy cytoplasm and wrinkled hyperchromatic nuclei
Stains with mucicarmine and Alcian blue stains
Osteoclast-like giant cell is a rare neoplasm (Fig. 11.13)
Classified in three types: osteoclastic, pleomorphic, and mixed.
However, since 2010 the World Health Organization has grouped them together as undifferentiated carcinoma with osteoclast-like giant cells.
Microscopic examination reveals osteoclast-like giant cells with glandular elements.
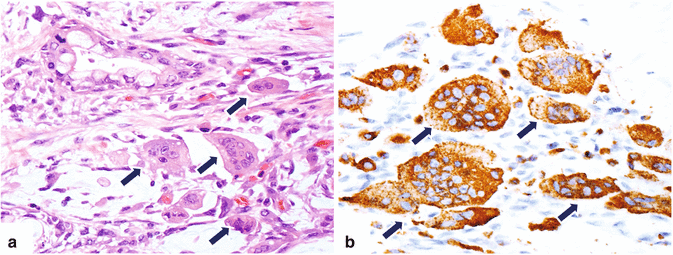
Fig. 11.13
Variant of pancreatic ductal adenocarcinoma, microscopic appearance. (a) Histological section (H&E, 20×) shows an osteoclast-like, cell-type, pancreatic adenocarcinoma characterized by large multinucleated giant cells (arrows) that are highlighted by immunohistochemistry with CD68 (b) (arrows) (immunoperoxidase 40×)
11.6 Clinical Presentation
The anatomic location of the pancreas is deep in the abdomen, thereby hindering early detection of this tumor.
Signs and symptoms occur late, and patients usually present with advanced disease.
Initial presentation varies according to tumor location.
Clinical Symptoms
Abdominal/back pain
Weight loss
Asthenia
Anorexia
Vomiting
Nausea
Diarrhea
Steatorrhea
Early satiety
Malaise
Fatigue
Sleep disturbance
Thrombophlebitis (Trousseau syndrome)
Clinical Signs of Advanced Disease
Jaundice
Ascites
Palpable abdominal mass
Hepatomegaly
Splenomegaly
Cachexia
Gastrointestinal bleeding (invasion of the duodenum, stomach, or colon)
Subcutaneous nodes in paraumbilical area (Sister Mary Joseph nodule)
Palpable mass in rectal pouch (Blumer’s shelf)
Virchow’s node (behind medial left clavicle)
Cervical lymph nodes
Practical Pearls
In up to 10 % of patients, new-onset diabetes may be the first clinical feature associated with this tumor.
Pancreatitis may also be the first signal of a pancreatic carcinoma, especially in the elderly, when there is no obvious cause such as gallstones or alcohol abuse.
Tumors in the head of the pancreas usually have an earlier presentation (jaundice).
Tumors localized in the uncinate process may be associated with epigastric or back pain without jaundice.
Tumors in the body and tail of the pancreas have a late presentation, often presenting with disseminated local or metastatic disease commonly found in the liver.
The presence of splenomegaly in a patient with pancreatic adenocarcinoma suggests the presence of splenic vein occlusion.
The spleen may have a normal size when there is simultaneous occlusion of the splenic vein and splenic artery produced by the tumor.
11.7 Laboratory Evaluation and Tumor Markers
Laboratory Evaluation
Typically nonspecific
Patient may be anemic
Patients with biliary obstruction and jaundice present with significantly elevated conjugated and total bilirubin, as well as an elevation of the alkaline phosphatase
General malnutrition markers: low serum albumin or low cholesterol
Tumor Markers
No specific tumor marker has been identified.
Most widely used serum marker is CA19-9 (0–37 U/ml normal range).
CA19-9 has low sensitivity and specificity.
Sensitivity is related to tumor size; limited sensitivity for small cancers.
Practical Pearls
Elevation of CA 19–9 may be associated with other malignancies of the biliary system, stomach, and/or colon.
It may also be elevated in benign conditions such as acute cholangitis, biliary obstruction, pancreatitis, hepatitis, and cirrhosis.
High levels of CA19-9 may help surgeons in selecting patients for exploratory laparoscopy.
CA19-9 can be used as a prognostic marker or an indicator of disease activity in patients with initially elevated levels.
Patients who lack the Lewis antigen, do not express CA19-9; even in large tumors.
The serum marker carcinoembryonic antigen (CEA) may also be elevated but generally has a limited use in the detection of this tumor.
11.8 Imaging
It is very difficult to establish the diagnosis of pancreatic carcinomas solely based on symptoms and signs.
Imaging studies are crucial to establish this diagnosis.
Despite the progress in imaging techniques, most cancers smaller than 1 cm remain undetected.
Contrast-enhanced computed tomography (CECT) is the method of choice for detecting and determining local extension of the tumor and assessing candidates for potential curative surgery.
The positive predictive value of CECT in determining unresectability is close to 100 %.
The negative predictive value is close to 30 %.
Endoscopic ultrasound (EUS)-guided biopsy is the most sensitive technique for the diagnosing and staging of pancreatic carcinomas.
Transabdominal ultrasound is initially used to identify the cause of abdominal pain or jaundice.
Magnetic resonance (MR) is indicated for equivocal cases by CECT.
11.8.1 Ultrasound (Figs. 11.14–11.20)
11.8.1.1 Findings
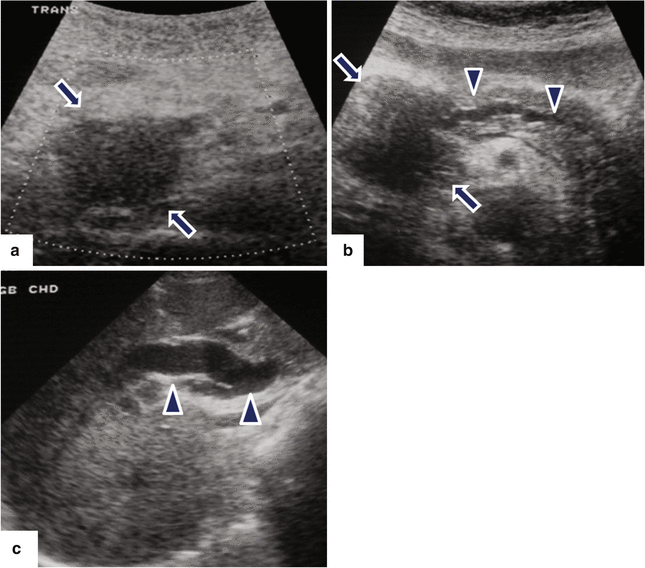
Fig. 11.14
Pancreatic ductal carcinoma of the pancreatic head on US. A 65-year-old male with epigastric pain and jaundice. Ultrasound transverse images (a–c) demonstrate a hypoechoic ill-defined mass in the pancreatic head (arrows) associated with obstruction of the pancreatic (b) and common bile (c) ducts (arrowheads)
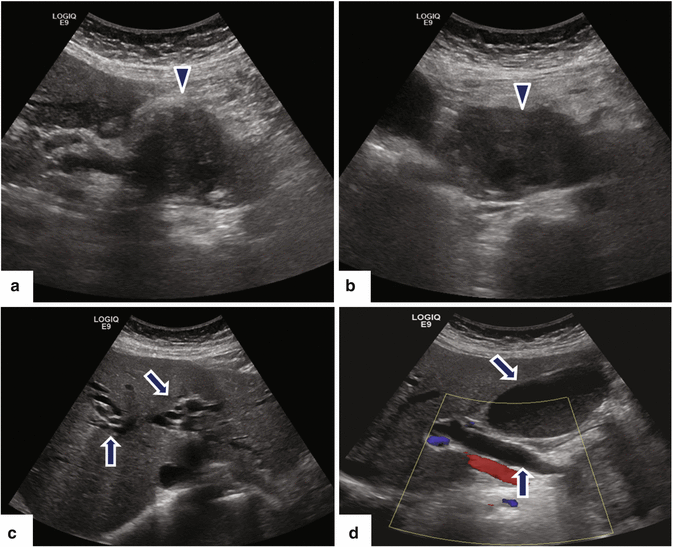
Fig. 11.15
Ductal carcinoma of the pancreatic head on US. A 64-year-old female with painless jaundice and weight loss. Ultrasound transverse (a–d) images show a large, hypoechoic mass (arrowheads) involving the pancreatic head causing obstruction of the pancreatic and common bile ducts and dilatation of the intrahepatic biliary system and gallbladder (arrows)
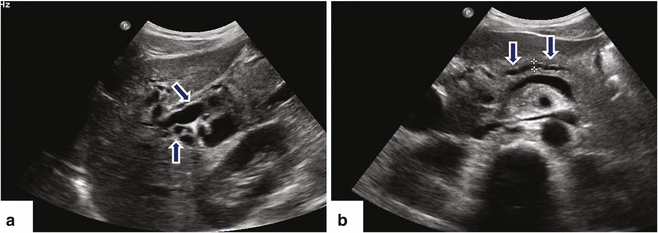
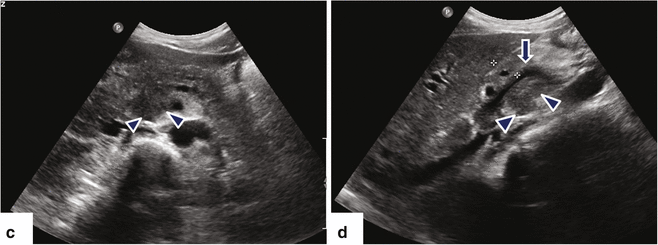
Fig. 11.16
Ductal carcinoma of the pancreatic head and uncinate process on US. A 40-year-old female with history of acute onset of jaundice and pruritus. Ultrasound transverse (a–c) and sagittal (d) images reveal obstruction of the common bile duct (a) (arrows) and pancreatic duct (b) (arrows) by a hypoechoic ill-defined mass (c, d) (arrowheads) involving the head and uncinate process. Note the partial encasement of the superior mesenteric artery (SMA) and the encasement and anterior displacement of the superior mesenteric vein (SMV) by this mass (d) (arrow)
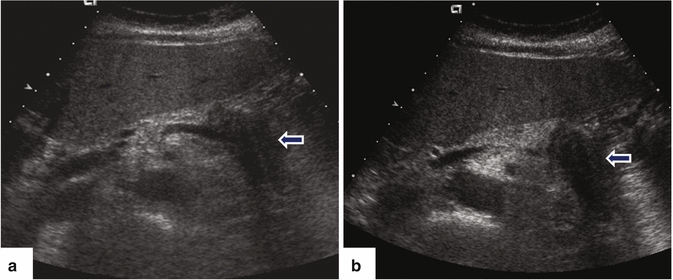
Fig. 11.17
Ductal carcinoma of the pancreatic body on US. A 64-year-old female with a history of diabetes mellitus diagnosed 5 years before, presenting with left upper quadrant abdominal pain, abdominal bloating, and decreased appetite. Ultrasound transverse (a, b) images show a poorly defined hypoechoic mass (arrows) involving the pancreatic body
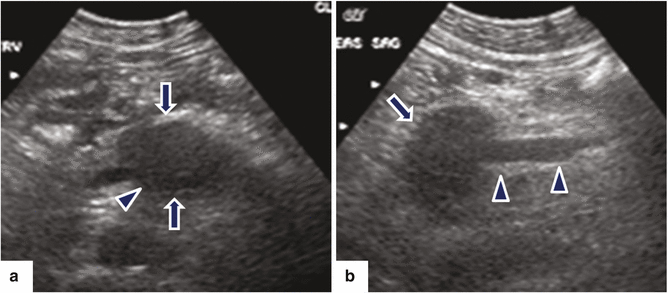
Fig. 11.18
Ductal adenocarcinoma of the pancreatic body with vascular invasion on US. A 53-year-old female with epigastric pain, malaise, and weight loss. Ultrasound transverse (a) and sagittal (b) images show a hypoechoic mass in the proximal body of the pancreas (arrows). Note the encasement of the proximal superior mesenteric vein (SMV) (arrowheads) by this mass
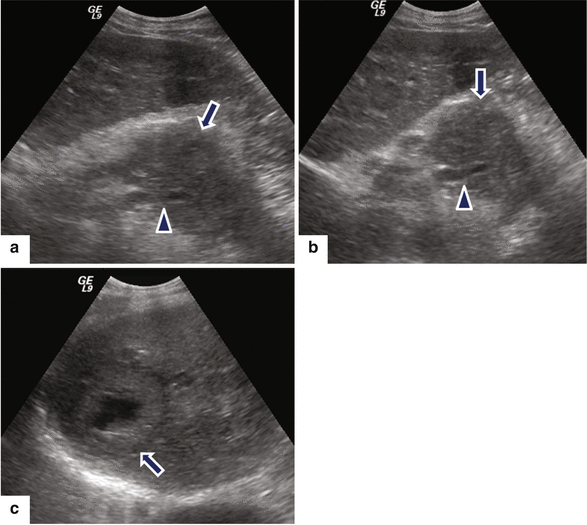
Fig. 11.19
Ductal adenocarcinoma of the pancreatic body with liver metastases on US. A 46-year-old male with history of back pain and abnormal liver function tests. Ultrasound transverse (a, b) images reveal a large, hypoechoic mass (arrows) in the pancreatic body encasing the splenic artery (arrowheads) and the presence of large, necrotic mass in the right lobe of the liver (c) (arrow)
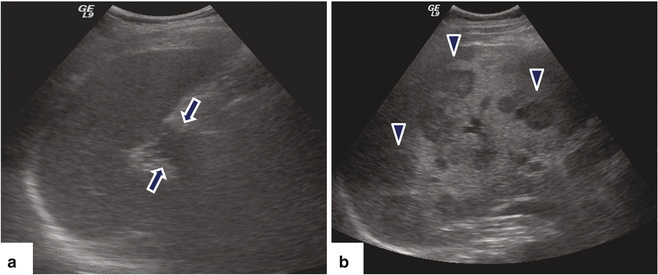
Fig. 11.20
Ductal adenocarcinoma of the pancreatic tail with liver metastases on US. A 67-year-old male with history of left upper quadrant and back pain. Ultrasound sagittal (a) and transverse (b) images demonstrate an infiltrative, hypoechoic mass (arrows) in the tail of the pancreas (a) and the presence of multiple, hepatic metastases (b) (arrowheads)
Transabdominal ultrasound (US)
Hypoechoic, ill-defined, avascular mass
Pancreatic duct dilatation and pancreatic parenchymal atrophy
Common bile duct obstruction (head mass)
Endoscopic ultrasound (EUS)
Homogeneous hypoechoic solid mass with ill-defined margins.
This method has the additional advantage of directing transduodenal or transgastric fine-needle aspiration biopsies.
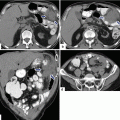
11.8.2 Computed Tomography (CT)(Figs. 11.21–11.60)
11.8.2.1 Findings
Plain CT
Isodense mass with convex borders
CECT
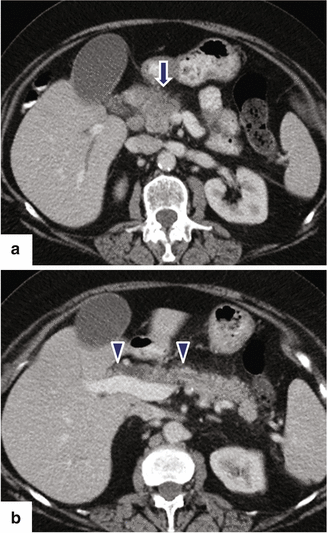
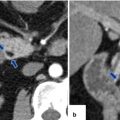
Fig. 11.21
Ductal adenocarcinoma of the pancreatic head on CT. A 76-year-old female with history of painless jaundice and weight loss. CECT axial (a, b) images demonstrate an infiltrative, low attenuation mass in the pancreatic head (a) (arrow). Note the extension of this mass into the peripancreatic fat and the associated dilatation of the pancreatic and common bile ducts (b) (arrowheads)
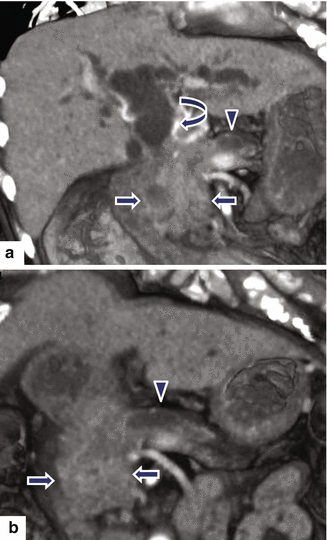
Fig. 11.22
Ductal adenocarcinoma of the pancreatic head on CT. A 74-year-old female with jaundice, abdominal pain, and weight loss. CECT coronal volume-rendered (a, b) images show a poorly defined, infiltrative mass (arrows) in the pancreatic head associated with obstruction of the distal common bile (curved arrow) and pancreatic ducts (arrowheads). Note the atrophy of the body and tail of the pancreas
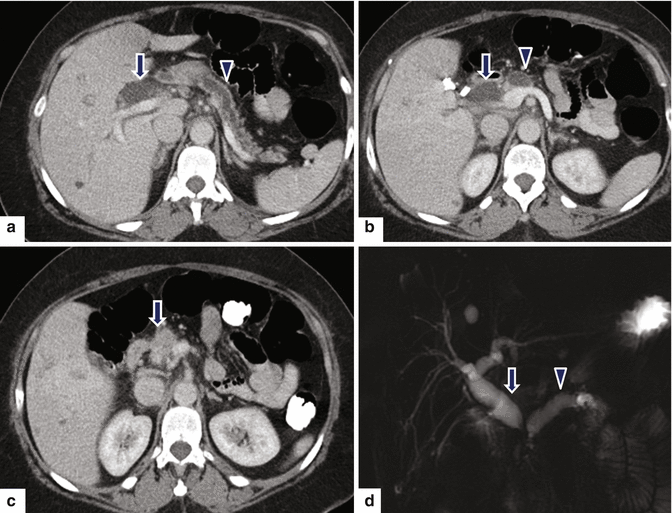
Fig. 11.23
Ductal adenocarcinoma of the pancreatic head on CT. A 73-year-old male with history of epigastric pain and discomfort. CECT axial (a, b) images show obstruction of the common bile duct (arrows) and pancreatic duct (arrowheads) at the same point, secondary to a small, ill-defined mass with subtle low attenuation (c) involving the pancreatic head (arrow). MRCP thick slab (d) image confirms the simultaneous obstruction of the pancreatic duct (arrowhead) and common bile duct (arrow) at the pancreatic head (“double duct” sign)
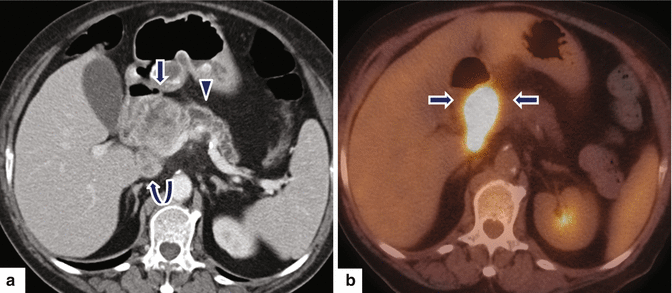
Fig. 11.24
Ductal adenocarcinoma of the pancreatic head on CT. A 78-year-old female with vague epigastric pain. CECT axial (a) image demonstrates a large, heterogeneous mass (arrow) involving the head of the pancreas causing obstruction of the distal pancreatic duct (arrowhead) and atrophy of the pancreatic parenchyma with the presence of an enlarged peripancreatic node (curved arrow). PET/CT axial (b) image demonstrates intense hypermetabolic activity in this mass
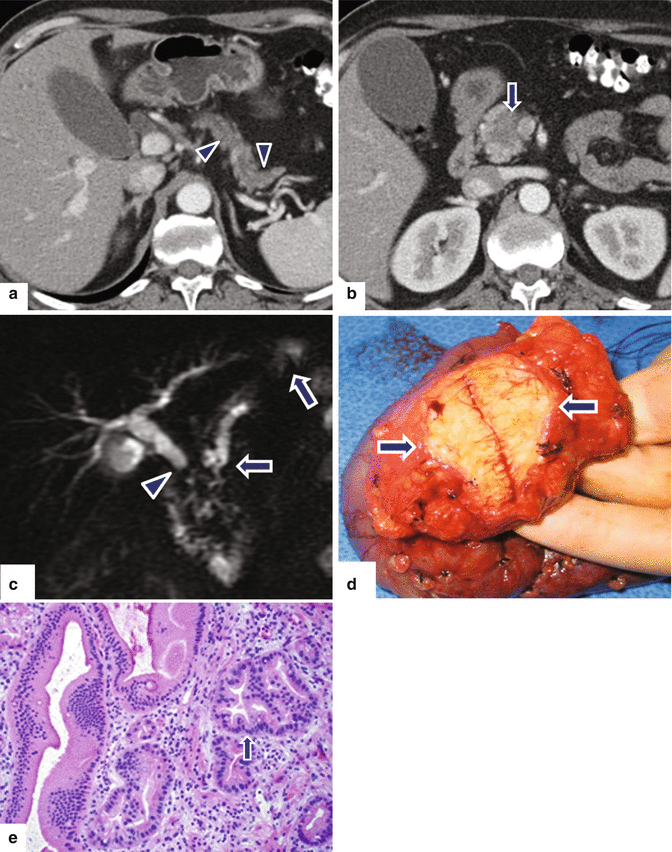
Fig. 11.25
Small nondeforming ductal adenocarcinoma on the pancreatic head on CT. A 72-year-old female with jaundice, abdominal pain, and weight loss. CECT axial (a, b) images demonstrate mild dilatation of the pancreatic duct, mild pancreatic atrophy (a) (arrowheads), and a nondeforming, low attenuation mass involving the pancreatic head (b) (arrow). MRCP thick slab (c) confirms the simultaneous obstruction of the common bile duct (arrowhead) and pancreatic duct (arrows) (“double duct” sign) up to the level of the pancreatic head. The patient underwent a pyloric-sparing pancreaticoduodenectomy. Photograph of the gross specimen (d) demonstrates an ill-defined, firm, white-tan mass (arrows). Histological section of the specimen (e) (H&E, 20×) shows a mass composed of malignant glands (arrow) and mild fibrosis
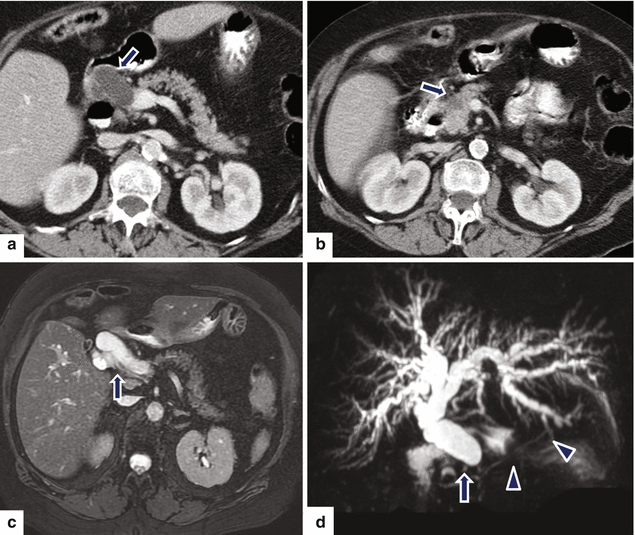
Fig. 11.26
Small nondeforming pancreatic ductal adenocarcinoma on CT. A 65-year-old male with history of pruritus, painless jaundice, and weight loss. CECT axial (a, b) images show abrupt cut-off of a dilated common bile duct at the superior aspect of the pancreatic head (a) (arrow) caused by a small hypoattenuating nondeforming mass in this location (b) (arrow). Fat-suppressed T2-weighted axial (c) and MRCP thick slab (d) images confirm the obstruction of the common bile duct with abrupt cutoff in the head of the pancreas (arrow). Note the normal appearance of the pancreatic duct (arrowheads) (d)
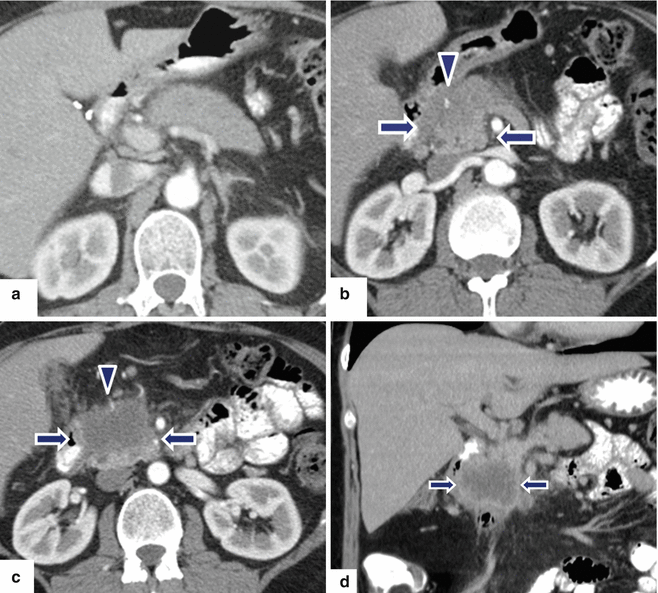
Fig. 11.27
Necrotic ductal adenocarcinoma of the pancreatic head on CT. A 53-year-old male with vague upper abdominal pain. CECT axial (a–c) and coronal (d) images demonstrate an infiltrative, low attenuation mass (arrows) involving the pancreatic head and encasing the gastroduodenal artery (arrowheads). Note the absence of dilatation of the pancreatic and common bile ducts associated with this large mass
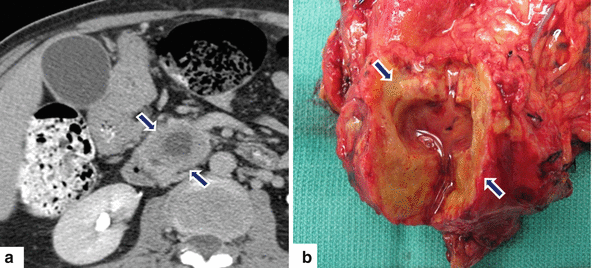
Fig. 11.28
Necrotic ductal adenocarcinoma of the pancreatic head on CT. A 71-year-old patient with history of weight loss and back pain. CECT axial (a) image shows a large round low attenuation mass with central necrosis (arrows) involving the pancreatic head. The patient underwent a pancreaticoduodenectomy. Photograph of the gross specimen (b) shows a firm, solid mass with central necrosis (arrows) containing clear fluid
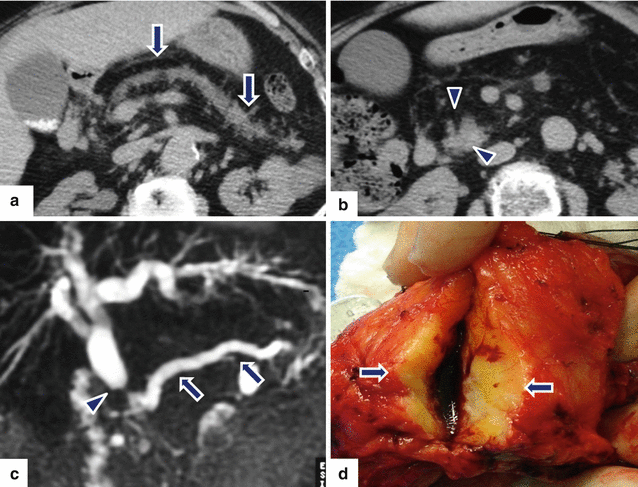
Fig. 11.29
Ductal adenocarcinoma of the pancreatic head associated with pancreatic lipodystrophy on CT. A 72-year-old male with history of weight loss and steatorrhea. CECT axial (a, b) images reveal marked dilatation of the pancreatic duct (arrows) caused by a vague pancreatic head mass (arrowheads). Note the diffuse fatty infiltration of the pancreatic parenchyma. MRCP thick slab (c) reveals obstruction of the common bile duct (arrowhead) and pancreatic duct (arrows) at the same point by this mass (“double duct” sign). Photograph of the gross specimen (d) reveals a solid, white-tan, ill-defined mass (arrows)
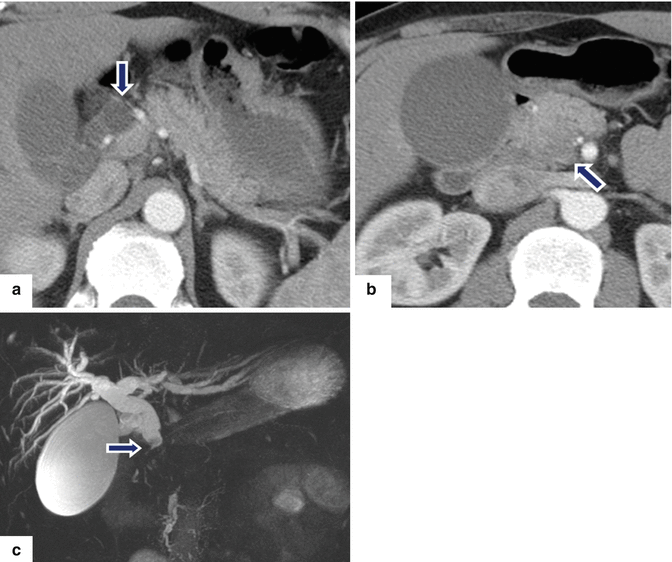
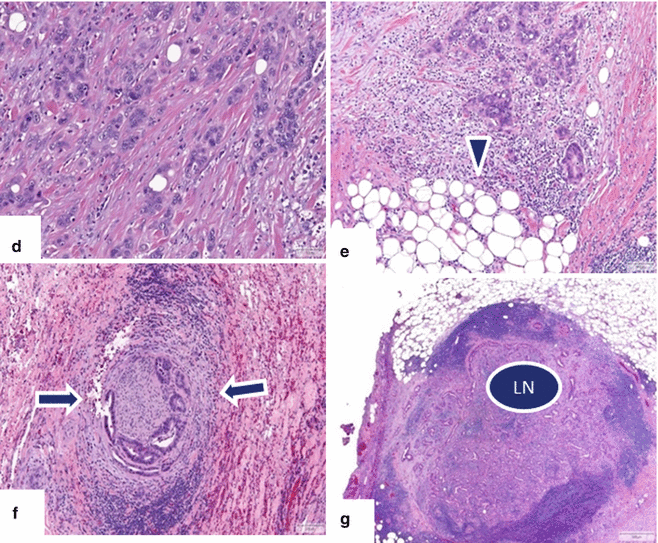
Fig. 11.30
Ductal adenocarcinoma of the pancreatic head and uncinate process on CT. A 52-year-old male with a 3 month history of painless jaundice. CECT axial (a, b) images show obstruction of the common bile duct (a) (arrow) caused by small, ill-defined mass of subtle low attenuation involving the head and uncinate process of the pancreas (b) (arrow). Note the dilatation of the gallbladder. MRCP thick slab (c) image confirms the obstruction of the CBD up to the level of the pancreatic head (arrow). Note the normal appearance of the pancreatic duct. The patient underwent a pancreaticoduodenectomy.Histological sections of the pancreatic mass (d, g) show a poorly differentiated pancreatic adenocarcinoma (d) extending into the peripancreatic tissue (e) (arrowhead) with perineural invasion (f) (arrows) and metastases to peripancreatic lymph node (LN) involvement (g) (H&E, 10×, 4×)
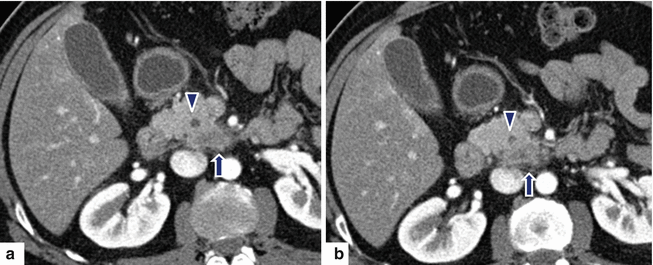
Fig. 11.31
Ductal adenocarcinoma of the uncinate process on CT. A 68-year-old female with history of back pain. CECT axial (a, b) images reveal an ill-defined, small mass involving the uncinate process of the pancreas infiltrating the peripancreatic fat (arrows). Note the association of this mass with mild dilation of the pancreatic duct (arrowheads)
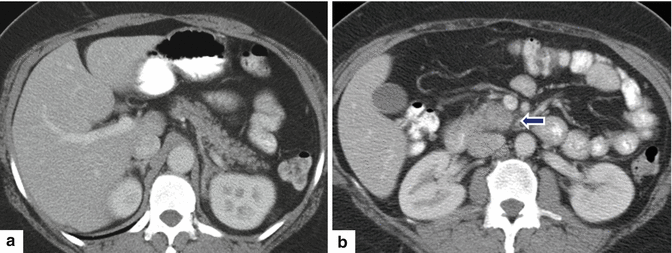
Fig. 11.32
Ductal adenocarcinoma of the uncinate process on CT. A 50-year-old female with a 3 month history of abdominal pain. CECT axial (a, b) images show a normal body and tail of the pancreas (a) and a rounded appearance of the uncinate process associated with mild peripancreatic fat stranding (b) (arrow). Findings suggestive of a pancreatic mass. This lesion was biopsied under endoscopic ultrasound guidance. Final diagnosis: well-differentiated ductal adenocarcinoma
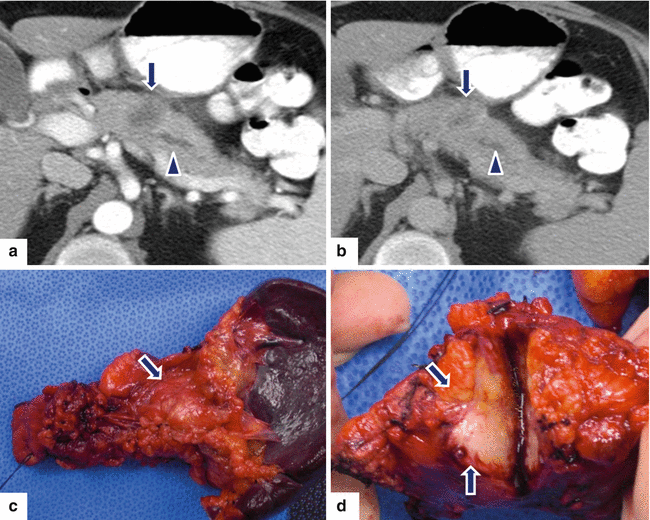
Fig. 11.33
Ductal adenocarcinoma of the pancreatic body on CT. A 59-year-old female with history of lower and mid-abdominal pain radiating to the back and new onset of diabetes. CECT axial arterial (a) and portal phase (b) images show a small ill-defined low attenuation mass (arrows) involving the pancreatic body causing obstruction of the pancreatic duct (arrowheads). Note the peripheral enhancement of this mass in the delayed phase (b) (arrow). The patient underwent a distal pancreatectomy and splenectomy. Photograph of the surgical specimen (c) shows the mass involving the pancreatic body (arrow). Photograph of the bivalved specimen (d) shows an infiltrative, solid, white-tan mass (arrows)
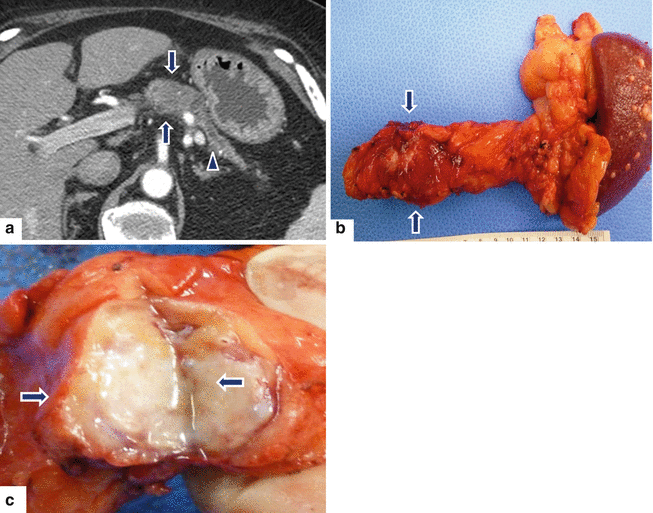
Fig. 11.34
Nondeforming ductal adenocarcinoma of the pancreatic body on CT. A 63-year-old male with history of epigastric pain and weight loss. CECT axial (a) image shows a well-defined, nondeforming low attenuation mass (arrows) involving the body of the pancreas associated with distal dilatation of the pancreatic duct and marked pancreatic atrophy (arrowhead). The patient underwent a distal pancreatectomy and splenectomy. Photograph of the surgical specimen (b) shows the pancreatic mass (arrows). Photograph of the bivalved specimen (c) shows a hard, white-gray mass with poorly defined margins
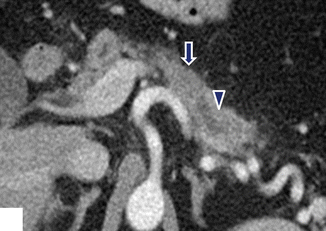
Fig. 11.35
Nondeforming, isodense ductal adenocarcinoma of the pancreatic body on CT. A 68-year-old female with history of intermittent back pain and diarrhea. CECT axial image demonstrates an isodense, nondeforming mass (arrow) involving the body of the pancreas associated with obstruction of the distal pancreatic duct (arrowhead)
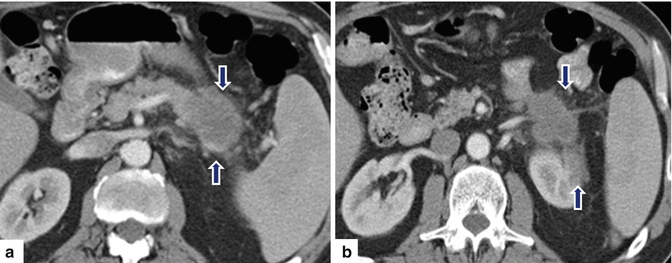
Fig. 11.36
Ductal adenocarcinoma of the body and tail of the pancreas on CT. A 49-year-old female with history of vague abdominal pain and weight loss. CECT axial (a, b) images demonstrate an infiltrative, low attenuation mass involving the body and tail of the pancreas extending posteriorly into the peripancreatic and perirenal fat (arrows)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree