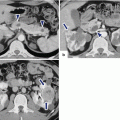
Fig. 10.1
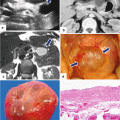
PNEN macroscopic appearance. Intraoperative and surgical speciments of four nonfunctional PNENs show (a) a small mass bulging on the serosal surface (arrows), (b) a small, round, purple mass (arrows), (c) a small mass with lobulated contours (arrows), and (d) a medium-sized, pale pink, firm mass with smooth borders (arrows)
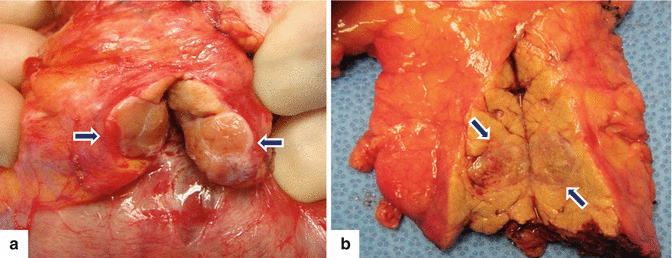
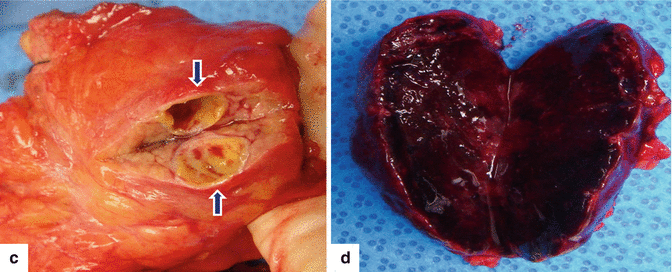
Fig. 10.2
PNEN macroscopic appearance – cross section. Photographs of four bivalved specimens demonstrate (a, b) light tan masses with a soft, fleshy homogeneous parenchyma (arrows), (c) a cystic mass with a thick capsule (arrows), and (d) a completely hemorrhagic mass with friable parenchyma
Usually solitary, well-demarcated, tan to pink soft tissue tumors.
The masses may be hard and gray-white when they exhibit fibrosis or amyloid deposition.
Sizes range from a few millimeters up to 20 cm; average range 1–5 cm.
Cystic degeneration: due to necrosis 5–10 %; containing clear or hemorrhagic fluid.
Vascular invasion: adverse prognostic factor.
Obstruction of the pancreatic duct (benign or malignant tumors).
Obstruction of the common bile duct (malignant tumors).
10.3.2 Microscopic Appearance (Fig. 10.3)
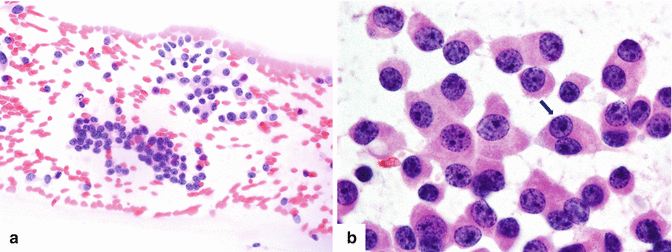
Fig. 10.3
Cytologic features: specimens demonstrate loosely cohesive groups (a) and isolated oval cells (b) with round, eccentrically located nuclei with the characteristic chromatin stippling (“salt-and-pepper”). The nuclei are mildly enlarged with well-defined nuclear borders. The arrow in (b) points to a binucleated cell (H&E, 10×, 100×)
Uniform polygonal cells arranged in a nested pattern.
Round to oval nuclei that have stippled, “salt-and-pepper” chromatin and scant eosinophilic granular cytoplasm.
Growth patterns: solid, trabecular, glandular, acinar, cystic, papillary, and angiomatoid.
Variable amounts of collagenous stroma and sclerosis.
Tumor differentiation refers to the extent of resemblance to the normal cellular counterpart (well or poorly differentiated).
Tumor grade refers to the degree of biologic aggressiveness (low, intermediate, and high grades) and is based on the mitotic count and the proliferative index by Ki-67 immunohistochemistry.
Tumor stage refers to the extent of spread of the tumor.
10.3.3 Immunohistochemical Endocrine Markers (Fig. 10.4)
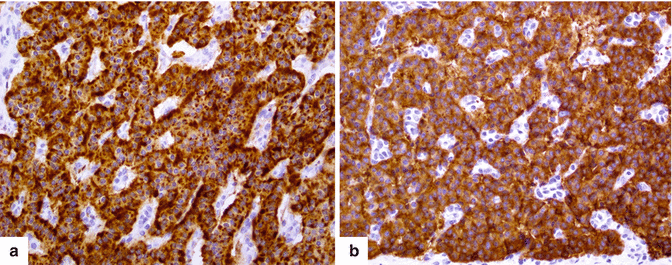
Fig. 10.4
Immunohistochemistry. The neurosecretory granules in neuroendocrine cells stain strongly positive for chromogranin (a) (20×) and synaptophysin (b) (20×) however, a faint or focal staining can be observed in poorly differentiated tumors
Chromogranin A: positive (protein found in secretory granules)
Synaptophysin: positive (membrane glycoprotein found in presynaptic vesicles)
Ki–67: used to assess the proliferative rate
Practical Pearls
In functional PNEN, the hormonal activity does not always correlate with immunohistochemical staining of the tumor.
Most nonfunctioning PNENs can show focal immunohistochemical staining for several peptides.
Features of Well-Differentiated PNEN
Tumor confined to pancreas
Usually do not metastasize
No necrosis
Low grade: low mitotic rate (less than 2 mitoses per 10 high-power fields) and low Ki-67 proliferative index (less than 3 % of Ki-67 staining)
Intermediate grade: intermediate mitotic rate (between 2 and 20 mitoses per 10 high-power fields) and intermediate Ki-67 proliferative index (3–20 % of Ki-67 staining)
Strong positivity for chromogranin and synaptophysin
Mutations of Common Oncogenes or Tumor-Suppressor Genes
K-ras
PTEN
RET
BRAF
Rb
CDKN2a/p16 DNA mismatch repairs and P53 are absent
Features of Poorly Differentiated PNEN
Infiltrative growth pattern.
Areas of necrosis can be seen.
Cells are small or large in size with irregular nuclei, scant cytoplasm, and abundant mitoses (>20 per 10 high-power fields).
Positive stain for chromogranin and synaptophysin (sometimes only focally).
High Ki-67 proliferative index (>20 %).
10.3.4 World Health Organization (WHO) Classification (Fig. 10.5)
The 2010 WHO classification recommends to use the term “neuroendocrine carcinoma” in the absence of metastases, only for poorly differentiated neoplasms (small cell carcinomas and large cell carcinomas) (Table 10.1).
Well-differentiated endocrine neoplasm
Low grade; grade 1
Intermediate grade; grade 2
Poorly differentiated endocrine carcinoma
High grade; grade 3
Classification is based on mitotic rate and cell proliferation (measured with the Ki–67 labeling index) (Fig. 10.6 ). Once the tumor shows cytologic features of high-grade carcinoma, it should be categorized as small cell carcinoma or large cell carcinoma.

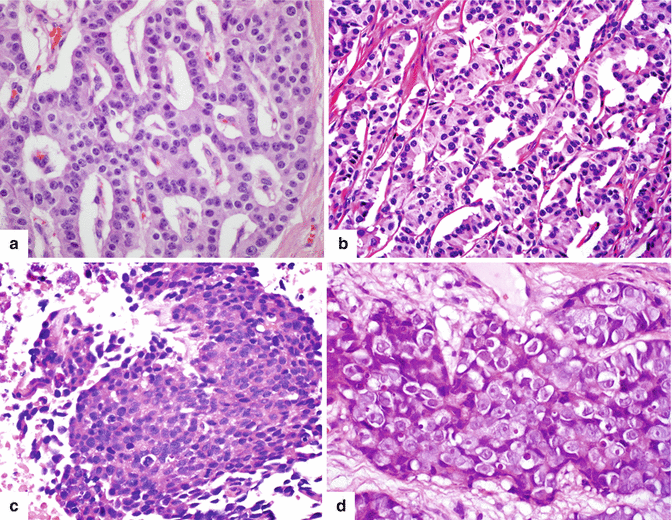
Fig. 10.5
Classification. PNENs are classified based on their degree of differentiation: well-differentiated low grade (a) (20×) and well-differentiated intermediate grade (b) (20×); or poorly differentiated carcinoma [small cell (c) (20×) or large cell neuroendocrine carcinoma (d) (60×)]. Well-differentiated tumors retain an organoid formation with a glandular pattern and abundant cytoplasm; intermediate-grade tumors display a more trabecular pattern; and poorly differentiated tumors are arranged in sheets of small or large cells with scant cytoplasm, prominent nucleoli, condensed chromatin and necrosis

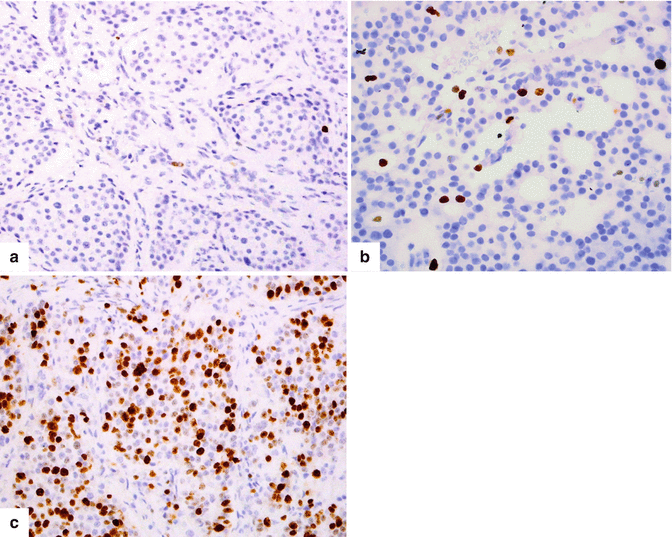
Fig. 10.6
Proliferative index. Immunohistochemistry for Ki-67 illustrates the proliferative rate of three different tumors: (a) <1 % expression grade 1 PNEN, (b) approximately 3 % expression grade 2 PNEN, and (c) >80 % expression grade 3 PNEN
Table 10.1
WHO Nomenclature for Pancreatic Neuroendocrine Neoplasms
Differentiation | ENETS/WHO 2010 Classifications and grading | Features | ||
|---|---|---|---|---|
Mitoses/10 HPF | Ki-67 proliferative index (%) | |||
Well-differentiated | Neuroendocrine neoplasm, grade 1 (G1) | <2 | <3 | |
Neuroendocrine neoplasm, grade 2 (G2) | 2–20 | 3–20 | ||
Poorly differentiated | Neuroendocrine carcinoma, grade 3 (G3) | Small cell carcinoma | >20 | >20 |
Large cell carcinoma | ||||
10.4 Nonfunctional PNEN
10.4.1 Epidemiology
Usually sporadic
More common than functional PNEN; representing up to 50–75 % of the cases
Most common PNEN in MEN-1 and VHL syndrome
Mean age of presentation: 55 years
Slight female predominance
60 % incidence of malignancy
Patients who have nonfunctional PNEN that are smaller than 2 cm are mostly cured by surgery
Many of these tumors secrete pancreatic polypeptide or other hormones without associated clinical symptoms.
May also produce an inert precursor hormone or low levels of an effective hormone.
10.4.2 Clinical Presentation
Nonfunctional PNENs remain clinically silent until they reach a noticeable size and provoke symptoms by tumor mass effect.
Associated Symptoms and Signs
Abdominal pain
Weight loss
Anorexia
Nausea
Abdominal mass
Jaundice (rare)
Pruritus
Practical Pearls
Silent, nonfunctional PNENs may eventually be discovered incidentally by abdominal imaging performed for another cause or during screening of a patient with MEN-1 syndrome.
Although nonfunctional PNENs secrete a number of substances such as chromogranin, neuron-specific enolase, pancreatic polypeptide, and ghrelin. They do not present clinically with a hormonal syndrome as compared to the functional neoplasms.
10.4.3 Laboratory Evaluation
Chromogranin A (CgA): most commonly secreted and measured hormone associated with all types of PNEN (normal value <93 ng/ml). Sensitivity of 50 %
Chromogranin A: useful marker for detecting residual or recurrent disease in treated patients
Practical Pearls
Chromogranin A can also be elevated in patients with carcinoid tumors, pheochromocytoma, neuroblastoma, medullary thyroid cancer, some pituitary tumors, and in amine precursor uptake and decarboxylation (APUD) tumors.
False-positive elevations of chromogranin A (CgA) are often seen in patients taking proton pump inhibitors or experiencing impaired hepatic or renal function or non-neuroendocrine tumors (i.e. testicular cancer).
10.4.4 Imaging
On average, nonfunctional PNENs are larger than functional PNENs
Average size: 5–6 cm
Usually solitary, except if they are associated with familial syndromes
Shape: round or oval, well-circumscribed or poorly defined margins
Calcifications and vascular invasion may be present
Even distribution throughout the pancreas
Diffuse involvement of the entire pancreas is rare
Metastases at the time of diagnosis (60–80 %), presenting as peripancreatic lymph nodes and/or liver masses
10.4.4.1 Ultrasound (US), Endoscopic Ultrasound (EUS), Intraoperative Ultrasound (IOUS) (Figs. 10.7–10.16)
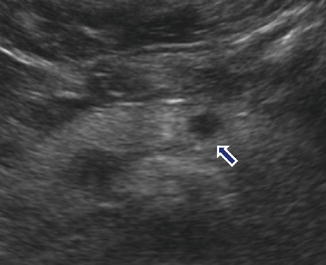
Fig. 10.7
Nonfunctional PNEN on US. A 41-year-old male with right upper quadrant pain. Incidental finding in an abdominal ultrasound. Transverse image shows a small, well-circumscribed, hypoechoic mass in the pancreatic body (arrow)
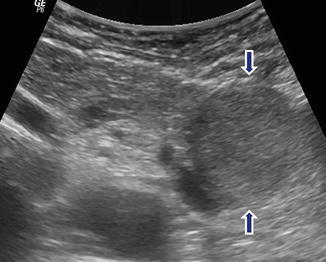
Fig. 10.8
Nonfunctional PNEN on US. A 62-year-old male with history of left quadrant pain. Transverse image reveals a round, well-defined, homogeneous, slightly hypoechoic mass in the tail of the pancreas (arrows)
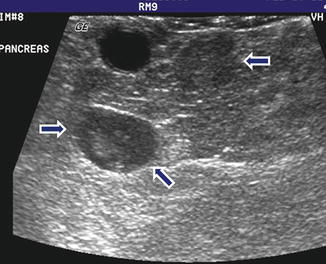
Fig. 10.9
Multiple nonfunctional PNENs on US. A 37-year-old male patient with history of MEN-1 syndrome. IOUS transverse image shows two small, well-defined hypoechoic masses in the pancreatic neck (arrows)
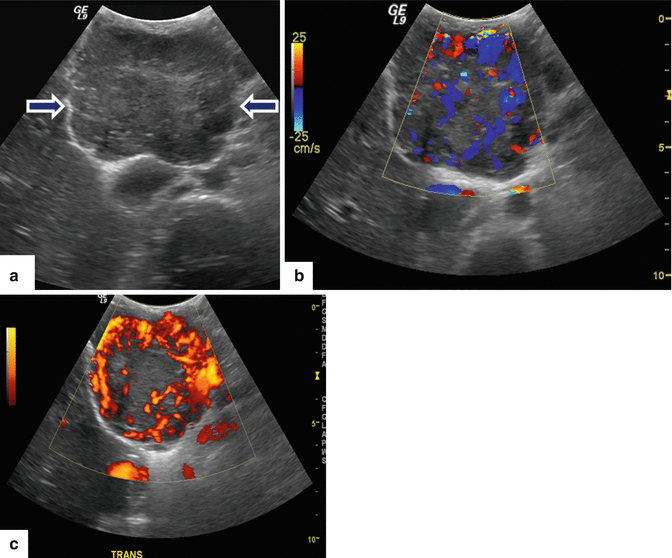
Fig. 10.10
Nonfunctional PNEN on US. An 18-year-old with history of von Hippel-Lindau disease. IOUS gray scale and color power Doppler transverse (a–c) images show a large homogeneous, hypoechoic, lobulated, hypervascular mass in the pancreatic head (arrows)
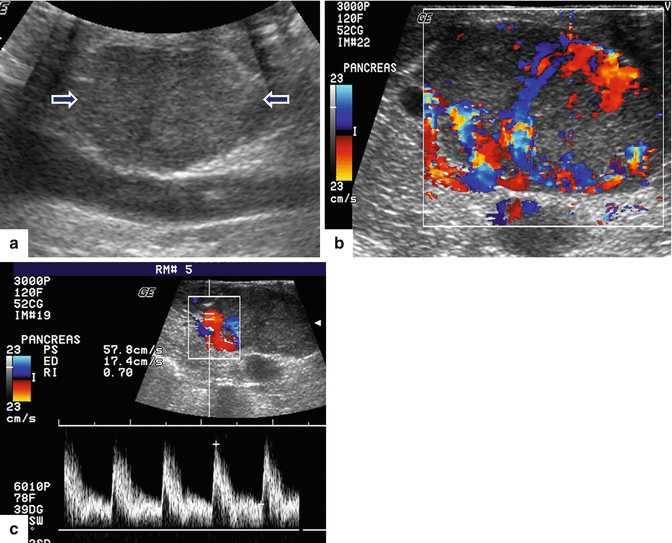
Fig. 10.11
Nonfunctional PNEN on US. A 50-year-old female patient with an incidental pancreatic finding. IOUS longitudinal gray scale (a) and color Doppler spectral (b, c) images show an ovoid, hypoechoic mass with prominent arterial supply in the pancreatic head anterior to the inferior vena cava (arrows)
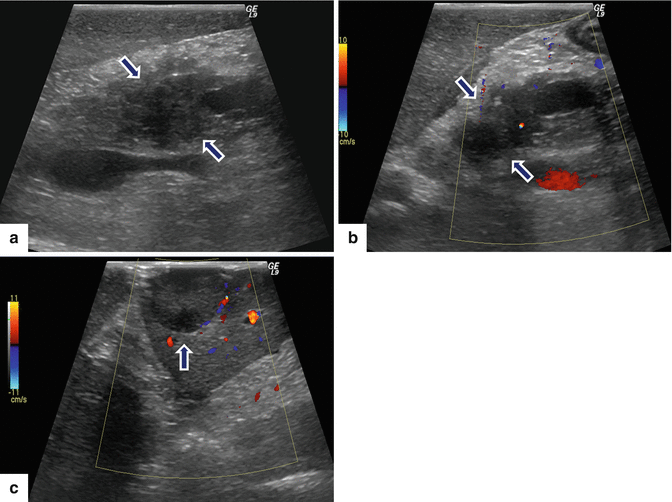
Fig. 10.12
Malignant nonfunctional PNEN on US. A 65-year-old female with history of epigastric pain and weight loss. Transverse (a) and sagittal (b, c) images demonstrate a hypoechoic mass with poorly defined margins in the pancreatic head (a, b) (arrows) and a hypoechoic, avascular metastatic deposit in the liver (c) (arrow)
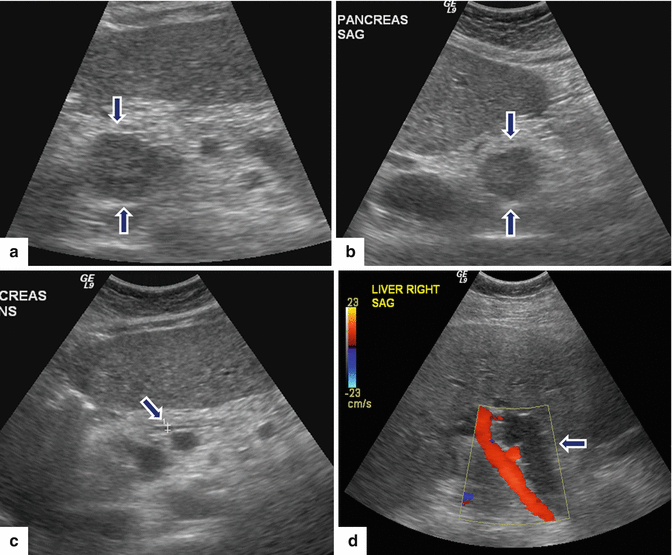
Fig. 10.13
Malignant nonfunctional PNEN on US. A 72-year-old male with history of epigastric pain and weight loss. Transverse (a) and sagittal (b) images show a round, hypoechoic mass, with ill-defined margins in the pancreatic head (arrows). Transverse images show mild dilatation of the pancreatic duct (c) (arrow) and obstruction of the common bile duct (d) (arrow) associated with this mass
Fig. 10.14
Cystic nonfunctional PNEN on US. A 41-year-old male with MEN-1 syndrome. IOUS transverse image demonstrates a small cystic mass with mild asymmetric wall thickening (arrows)
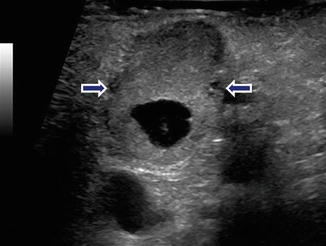
Fig. 10.15
Complex nonfunctional PNEN on US. A 53-year-old female with an incidental finding on a CT of the chest. IOUS transverse image reveals a complex mass with solid and cystic components in the pancreatic body (arrows)
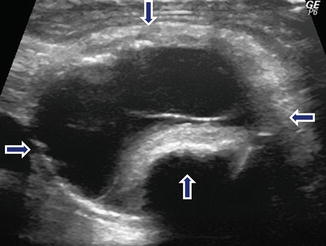
Fig. 10.16
Complex nonfunctional PNEN on US. A 72-year-old male with abdominal pain. IOUS transverse image displays a complex septated cystic mass (arrows) with a coarse calcification in the pancreatic body
Findings
Well-circumscribed, round or oval, hypoechoic mass with smooth margins
Color Doppler: hypervascular or hypovascular
Signs of Malignancy
Ill-defined margins
Enlarged peripancreatic nodes
Liver metastases: hyperechoic or hypoechoic
Pancreatic duct obstruction (rare)
Biliary obstruction (rare)
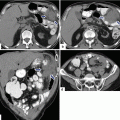
10.4.4.2 Computed Tomography (CT) (Figs. 10.17–10.43)
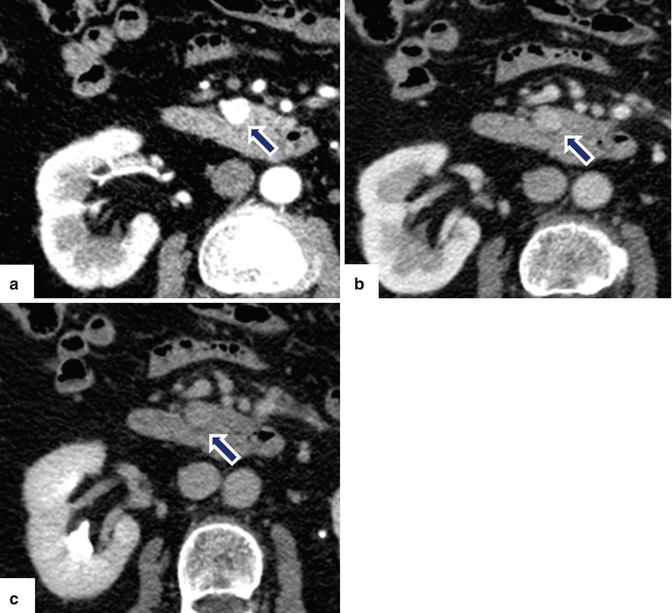
Fig. 10.17
Small nonfunctional PNEN in the pancreatic head on CECT. A 45-year-old female with incidental finding. CECT arterial (a), portal (b), and delayed phase axial (c) images show small, hypervascular mass in the pancreatic head (arrows). Note that in the different phases, this mass matches the enhancement pattern of the aorta
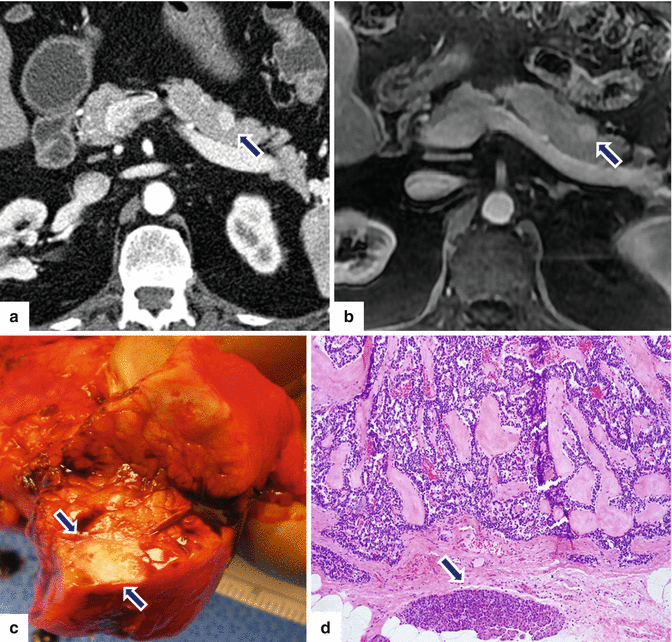
Fig. 10.18
Small nonfunctional PNEN in the pancreatic body on CT and MR. A 67-year-old male with history of nausea and vomiting. Incidental finding on an abdominal CT. CECT arterial axial (a) and contrast-enhanced fast spin gradient echo T1-weighted axial (b) images demonstrate a small, well-circumscribed, hypervascular mass in the pancreatic body (arrows). The patient underwent a distal pancreatectomy. Photograph of the bivalved specimen (c) demonstrates a small, ill-defined, yellow-tan mass (arrows). Microscopic examination (d) reveals a well-differentiated neuroendocrine neoplasm with hyalinized trabecular areas and a pseudocapsule adjacent to a small residual pancreatic lobule (arrow)
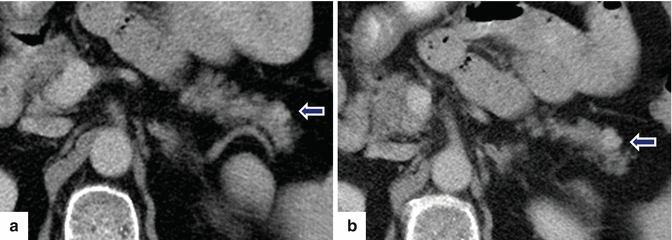
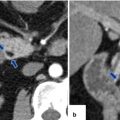
Fig. 10.19
Small nonfunctional PNEN in the pancreatic tail on CT. A 45-year-old female with incidental finding. CECT axial (a, b) images reveal an 8 mm lobulated hypervascular mass in the pancreatic tail (arrows)
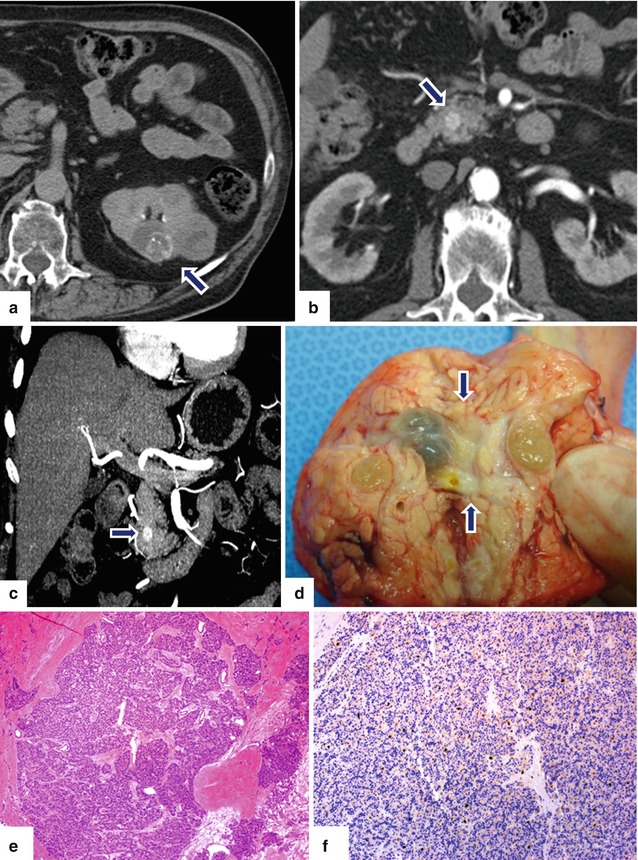
Fig. 10.20
Small nonfunctional PNEN versus pancreatic metastases on CT. A 56-year-old male with history of renal carcinoma. CECT performed 3 years prior (a) revealed a complex bilobulated renal cell carcinoma of the left kidney (arrow). The patient underwent a partial nephrectomy. Follow-up abdominal CECT axial (b) and coronal MIP (c) images show a small hypervascular mass in the head of the pancreas (arrows). Differential diagnosis included a metastatic deposit from the previous renal carcinoma versus a nonfunctional PNEN. Patient underwent a pancreaticoduodenectomy. Photograph of the gross specimen (d) shows a yellow-greenish fleshy tumor (arrows). Histological sections show (e) (H&E, 4×) a well-differentiated neuroendocrine neoplasm with a very low Ki-67 proliferative index of < 1% (f) (immunohistochemistry, 10×)
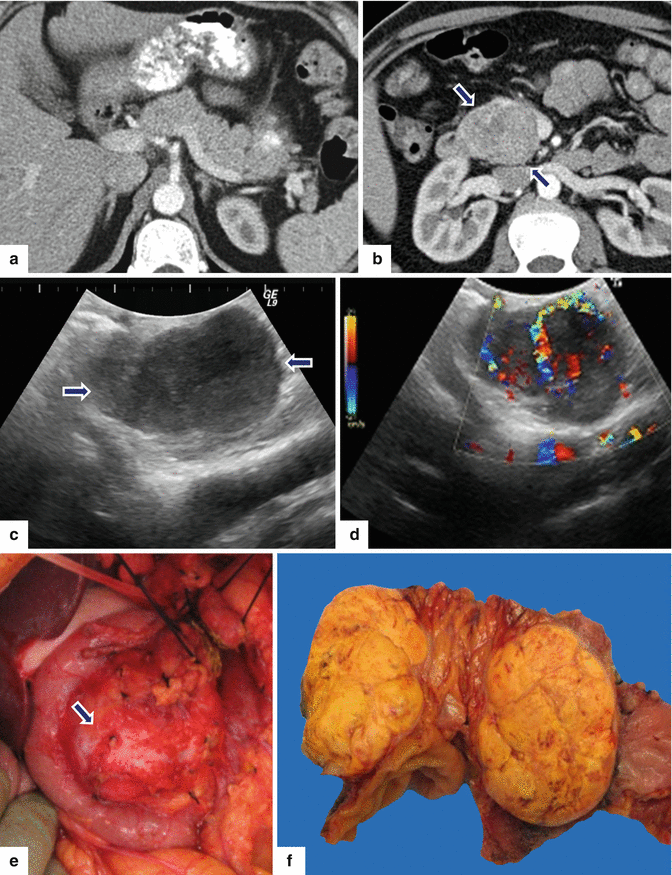
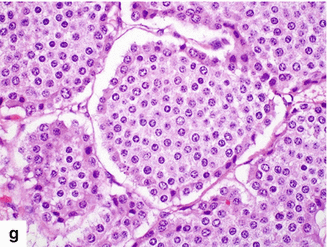
Fig. 10.21
Large nonfunctional PNEN in the pancreatic head on CT. A 44-year-old female complaining of right upper quadrant pain. CECT axial (a, b) images demonstrate a large hypervascular, heterogeneous mass in the pancreatic head. Note the absence of dilatation of the pancreatic duct and common bile duct associated with this mass. IOUS gray scale and color Doppler transverse (c, d) images demonstrate a well-differentiated, hypoechoic, partially vascular, solid mass. Intraoperative photograph (e) demonstrates a large pancreatic head mass abutting the duodenum (arrows). Photograph of the bivalved mass (f) demonstrates a fleshy, yellow, soft mass. Histological section (g) (H&E, 40x) shows cells with abundant eosinophilic cytoplasm arranged in a nesting pattern. The nuclei display a “salt-and-pepper” appearance of the chromatin
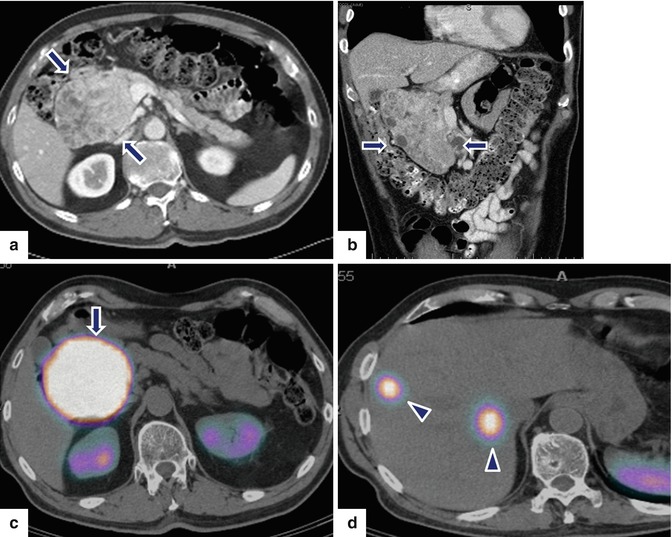
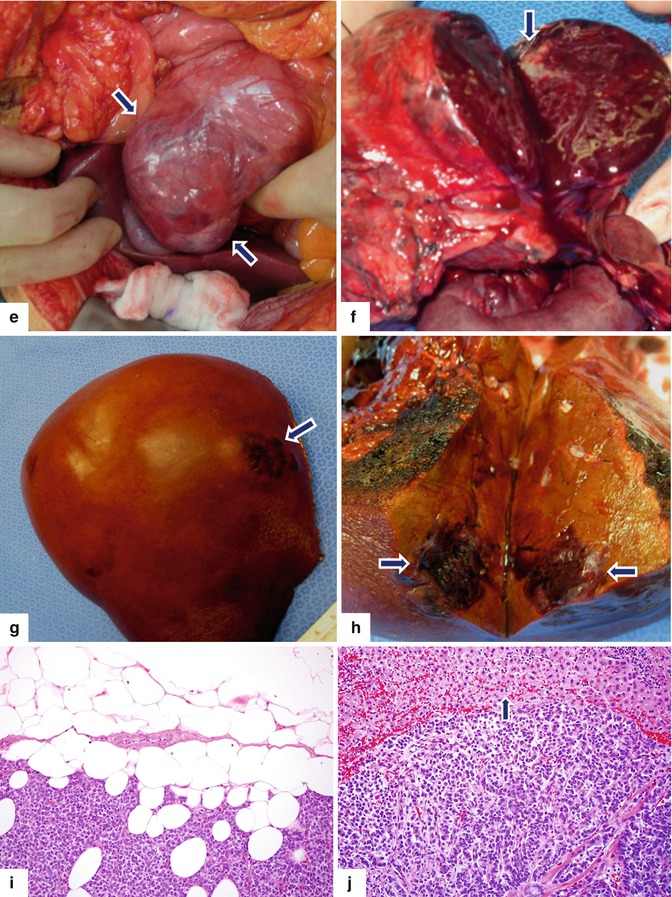
Fig. 10.22
Large nonfunctional PNEN in the pancreatic head on CECT and octreotide scan. A 69-year-old male undergoing abdominal CT workup for an aortic bruit was found to have a mass in the head of pancreas and two small masses in the right lobe of the liver. CECT axial (a) and coronal (b) images show a large, hypervascular, heterogeneous mass involving the pancreatic head (arrows). Octreotide scan (c, d) shows intense uptake of the radiotracer by this mass (arrows) and two hot spots in the right lobe of the liver (arrowheads). Patient underwent a pancreaticoduodenectomy and a right lobe hepatectomy. Intraoperative photograph (e) shows a large, bilobulated, red mass with smooth serosal surface that is located in the pancreatic head (arrows). Photograph of the bivalved specimen (f) shows a large tumor mass with red, hemorrhagic parenchyma and peripheral scarring (arrow). Photograph of the resected right lobe of the liver (g) shows a round area with irregular raised borders (arrow) located in segment VIII. A cross section of the mass reveals (h) an ill-defined, mahogany-brown tumor (arrows). Histological sections showed an intermediate-grade (grade 2) neuroendocrine carcinoma of the pancreas extending into the peripancreatic adipose tissue (i) and metastatic carcinoma to two lymph nodes and to the liver (j) (arrow) (H&E, 20×)
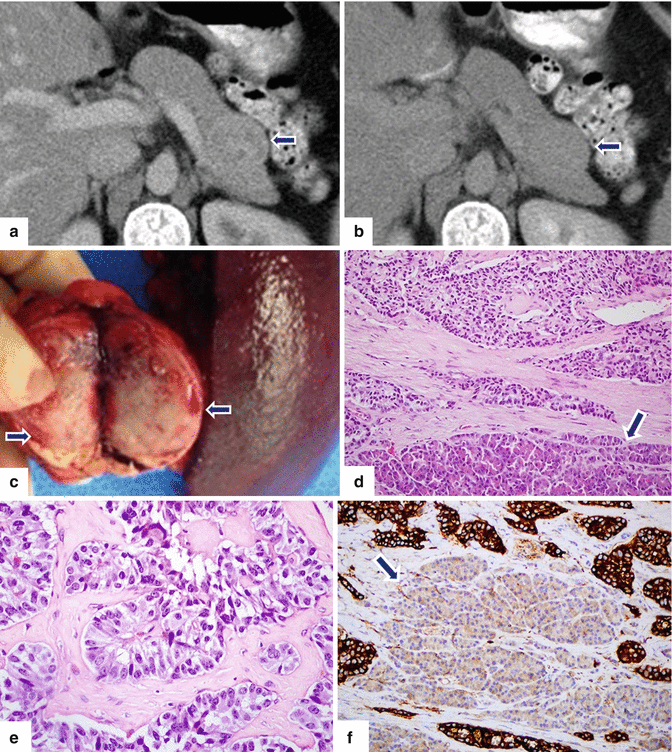
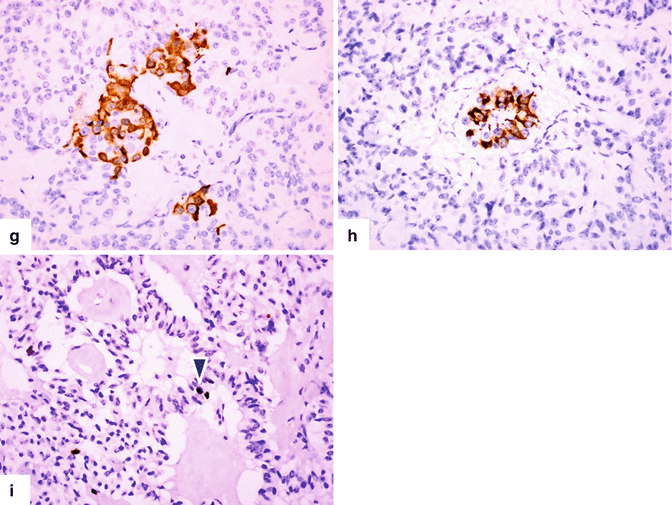
Fig. 10.23
Large nonfunctional PNEN in the pancreatic tail on CECT. A 37-year-old female with history of pelvic pain who underwent an abdominal CT that showed an incidental finding in the pancreas. CECT axial arterial (a) and delayed phase (b) images demonstrate a round hypervascular mass with heterogeneous attenuation involving the tail of the pancreas (arrows). Note that this mass became isodense with the rest of the pancreas in the delay phase (arrowheads). A distal pancreatectomy and splenectomy was performed on this patient. Photograph of the bivalved specimen (c) shows a soft, tan, and partially hemorrhagic tumor (arrows). Histopathological examination demonstrated a well-differentiated neuroendocrine neoplasm (grade 1) infiltrating the pancreas (d) (arrow) with a nesting and trabecular pattern (e) (H&E, 50×). Tumor cells are strongly positive for synaptophysin (f) (Immunohistochemistry, 20×). Note the residual pancreatic lobule surrounded by tumor cells (f) (arrow). The tumor cells were focally positive for glucagon (g) and insulin (h), Ki-67 proliferative index was <3 % (i) (arrowhead) (Immunohistochemistry, 50×)
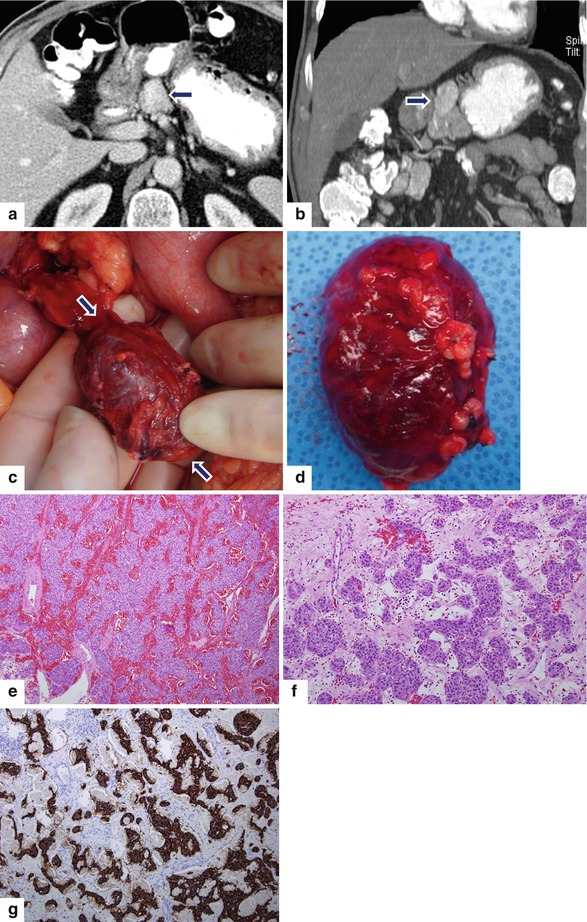
Fig. 10.24
Exophytic PNEN on CT. A 77-year-old male with history of diabetes and recent 12 lb weight loss. CECT axial (a) and coronal (b) images show an exophytic, ovoid, hypervascular, homogeneous mass arising in the neck of the pancreas (arrows). Intraoperative and gross photographs (c, d) show an oval, well-circumscribed, pedunculated pancreatic mass (arrows) with a smooth serosal surface. Histological sections show highly vascular areas with abundant hemorrhage (e) (H&E, 10×) and areas with tumor cells arranged in an organoid pattern (f) (H&E, 20×). Mitoses were not identified. Immunohistochemistry for synaptophysin (g) highlights the tumor cells
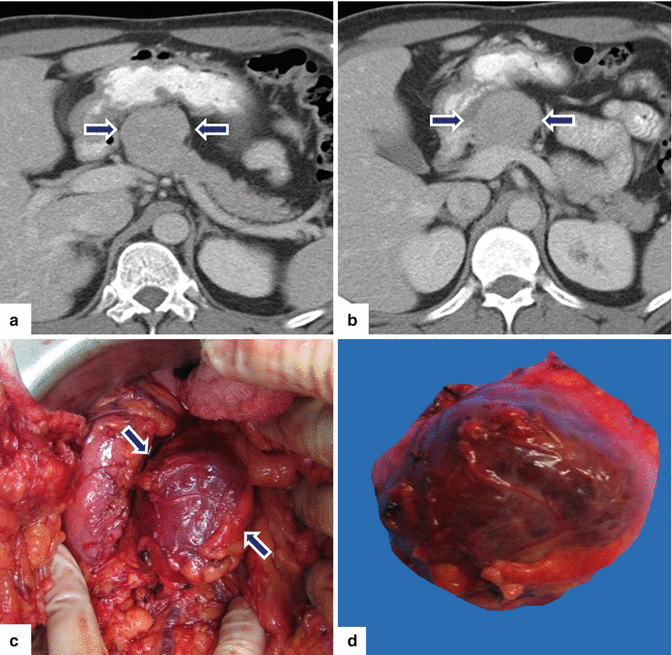
Fig. 10.25
Exophytic PNEN on CT. A 38-year-old male with history of small bowel neuroendocrine tumor resected 8 years prior. Annual CT surveillance found an incidental pancreatic mass. CECT axial (a, b) images reveal an exophytic, isodense mass arising in the pancreatic neck (arrows). Intraoperative photograph (c) reveals a round to oval, purple mass (arrows). Patient underwent a central pancreatectomy. Photograph of the gross specimen (d) demonstrates the oval mass with a smooth serosal surface. Final pathology: well-differentiated PNEN
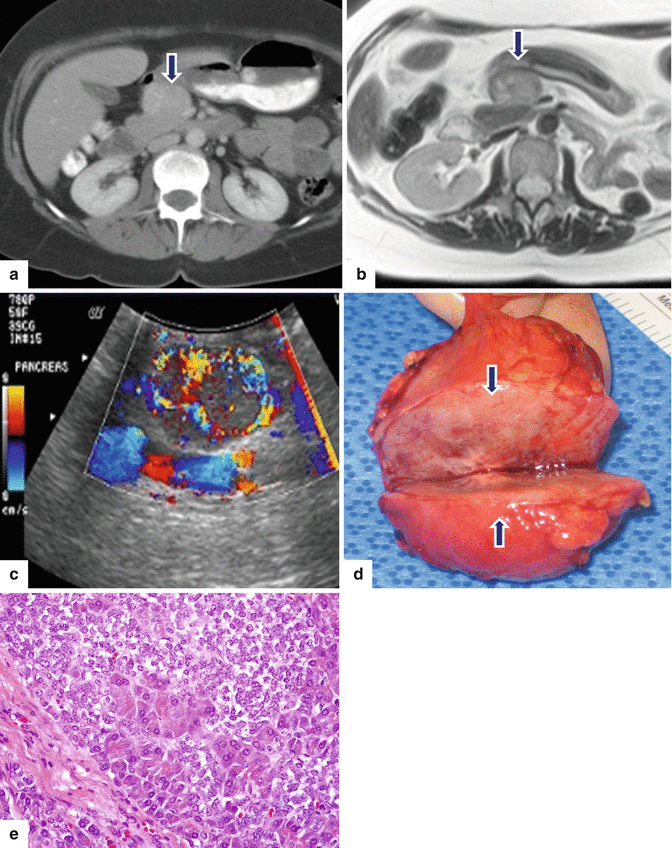
Fig. 10.26
Exophytic PNEN on CT and MR. A 50-year-old female with history of renal stones. Surveillance abdominal CT demonstrated a pancreatic mass. CECT axial (a) image shows a homogeneous, exophytic, hypervascular mass arising from the anterior aspect of the pancreatic head (arrow). T2WI axial (b) image confirms the presence of an exophytic pancreatic mass with heterogeneous signal intensity (arrow). IOUS axial (c) image shows a hypervascular pancreatic mass. Photograph of the bivalved specimen (d) demonstrates a round, well-circumscribed mass with a fleshy appearance and a central stellate scar (arrows). Microscopic examination demonstrated a well-differentiated neuroendocrine neoplasm, grade 2. The tumor cells are arranged in sheets (e) (H&E, 40×)
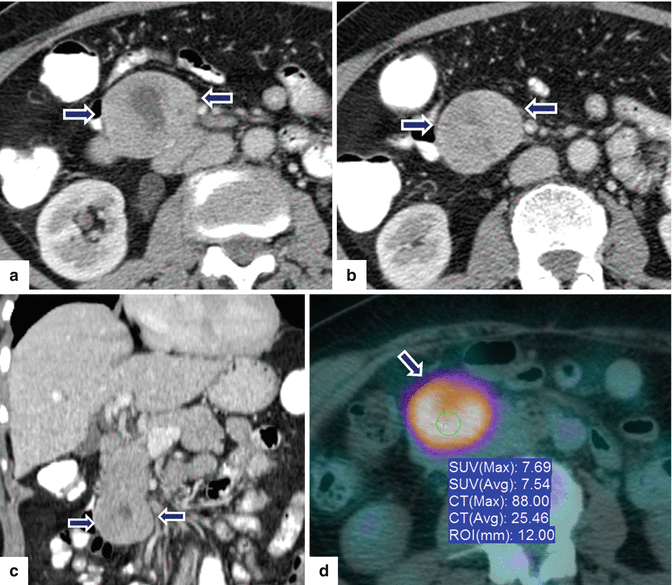
Fig. 10.27
Pedunculated necrotic PNEN on CT and PET/CT. A 71-year-old female with history of mild epigastric pain. CECT axial (a, b) and coronal (c) images show a large round exophytic, hypervascular mass with central necrosis arising from the inferior aspect of the pancreatic head (arrows). PET/CT axial (d) image shows avid FDG uptake by this mass (arrow)
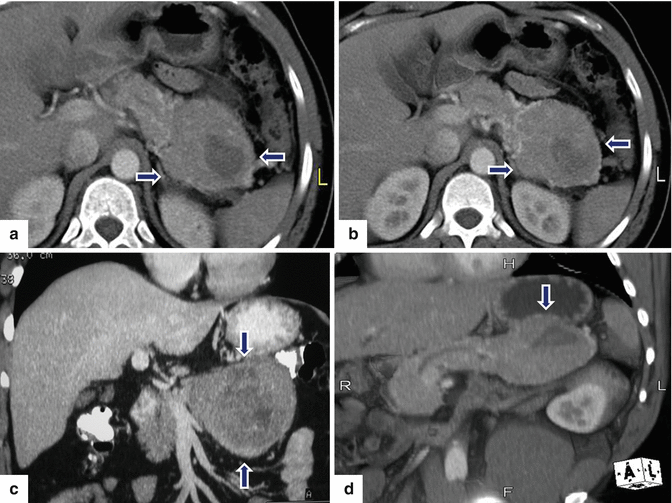
Fig. 10.28
Partially necrotic PNEN on CT. A 41-year-old male complaining of abdominal pain. CECT axial (a, b) and coronal (c, d) images demonstrate a well-defined, oval, heterogeneously attenuating mass with central necrosis involving the tail of the pancreas (arrows). Patient underwent a distal pancreatectomy and splenectomy. Final pathology: large, well-differentiated pancreatic neuroendocrine neoplasm, grade 1, with mild nuclear atypia without mitosis
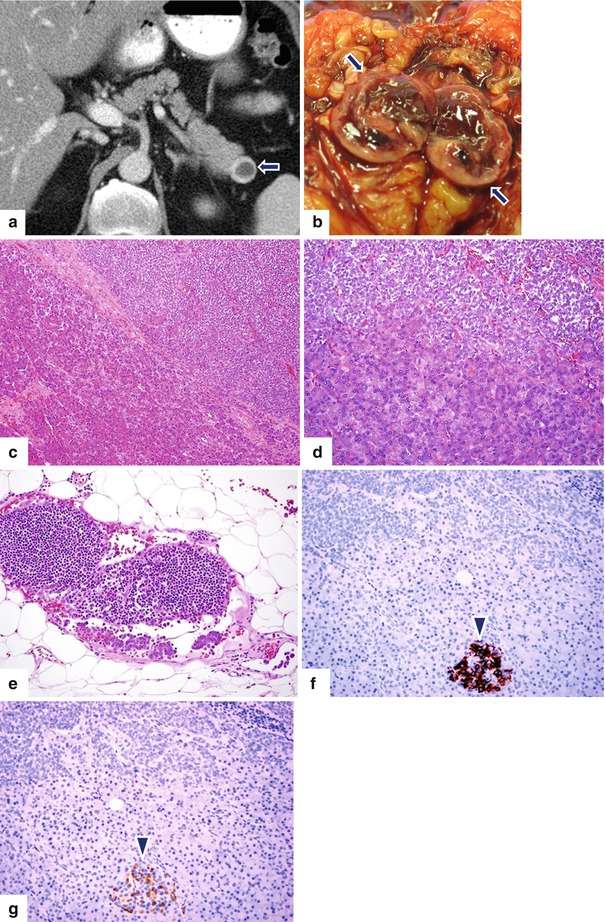
Fig. 10.29
Cystic PNEN on CT. A 62-year-old male with history of prostatic carcinoma. Surveillance abdominal CT found an incidental mass in the pancreas. CECT axial (a) image demonstrates a round cystic mass with a thick peripheral enhancing rim (arrow). Patient underwent a distal pancreatectomy and splenectomy. Photograph of the bivalved gross specimen (b) shows a friable mass with a thick periphery wall (arrows) and central hemorrhage. Histologic examination revealed a well-differentiated neuroendocrine carcinoma (c, d) (H&E 10×, 20×) with metastasis to one peripancreatic lymph node (e) (H&E, 20×). Immunohistochemistry for insulin (f) (20×) and glucagon (g) (20×) is negative in the tumor cells and highlights the beta and alpha cells of a residual islet of Langerhans (f) (arrowhead) respectively. The tumor was nonfunctional
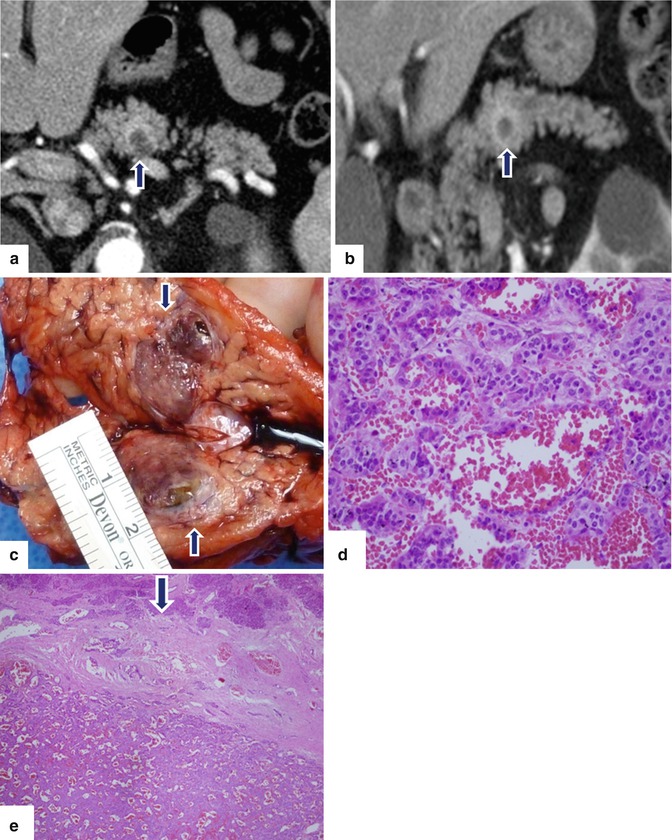
Fig. 10.30
Cystic PNEN on CT. A 58-year-old male with vague periumbilical pain. Abdominal CT found an incidental mass in the pancreas. CECT axial (a) and coronal (b) images reveal a cystic mass with a thick enhancing capsule (arrows). Photograph of the bivalved gross specimen (c) shows a partially hemorrhagic, ill-defined mass (arrows). Histological sections show (d) (H&E, 50×) areas of tumor cells with trabecular arrangement and hemorrhage. Solid areas (e) (H&E, 10×) show cells with focal pseudoglandular arrangement and dense fibrotic areas (e, arrow). Final diagnosis: well-differentiated, neuroendocrine neoplasm, grade 1.
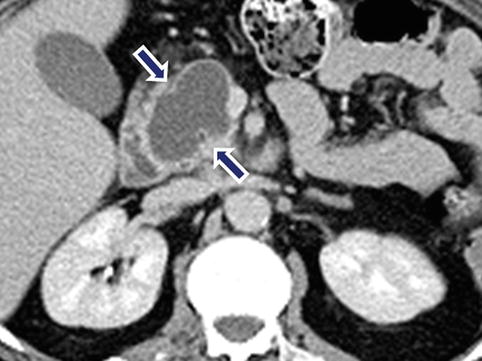
Fig. 10.31
Cystic PNEN on CT. A 46-year-old male with epigastric discomfort. CECT axial image shows an ovoid cystic mass with a thin enhancing wall involving the pancreatic head (arrows). Final pathology: well-differentiated neuroendocrine neoplasm
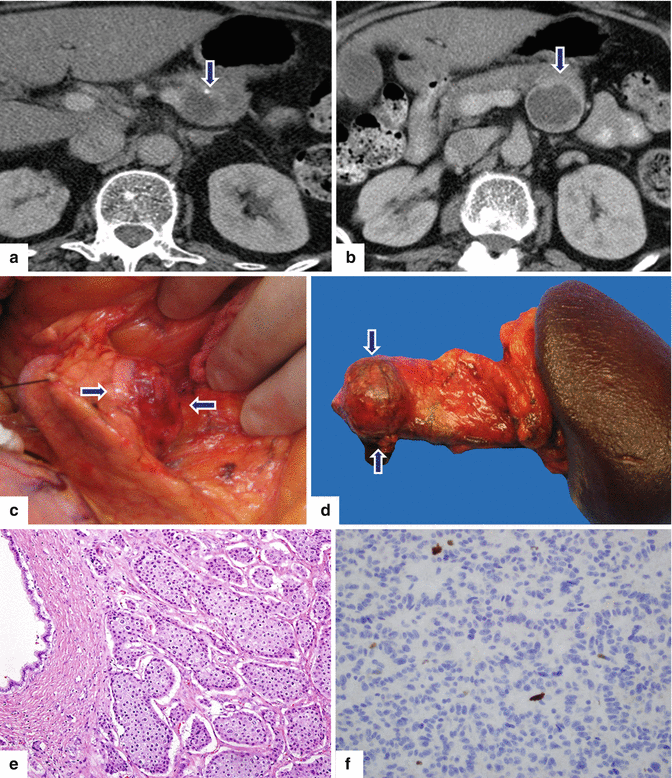
Fig. 10.32
Cystic nonfunctional PNEN on CT. A 61-year-old female with history of rheumatoid arthritis. Incidental pancreatic mass was found during an abdominal CT examination. CECT axial (a, b) images demonstrate an exophytic, round, cystic mass with a punctate calcification and enhancing wall involving the body of the pancreas (arrows). Intraoperative photograph (c) demonstrates an exophytic mass (arrows). Patient underwent a distal pancreatectomy and splenectomy. Photograph of the gross specimen (d) demonstrates a red, round mass with smooth serosal surface (arrows). Histological sections of the mass show a well-differentiated, neoplasm, grade 1, (e) (H&E, 20×) with a nesting pattern and a low Ki-67 proliferative index (f) (Immunohistochemisty, 40×)
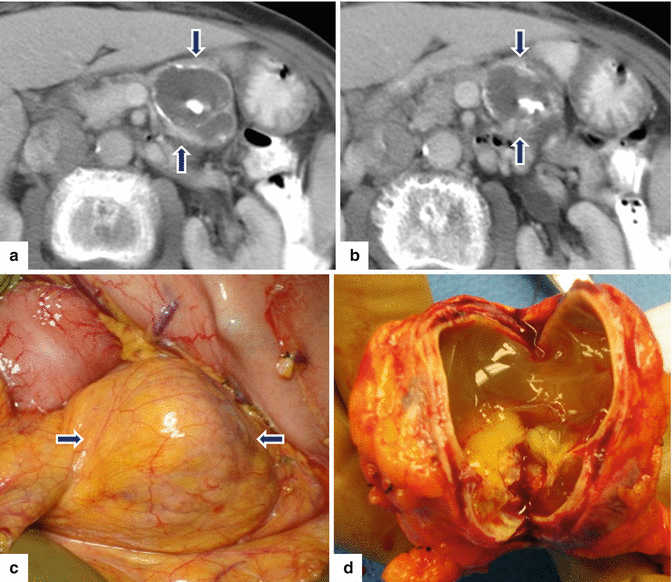
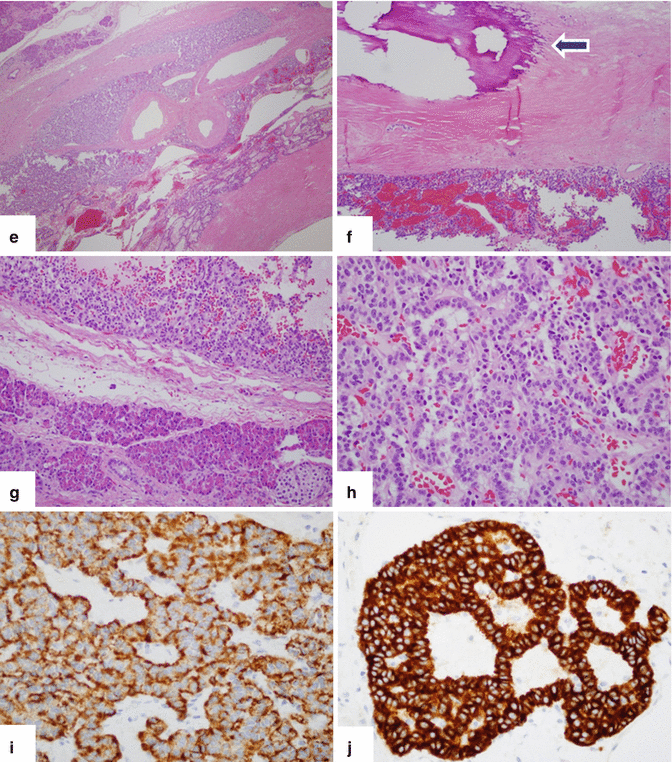
Fig. 10.33
Complex nonfunctional PNEN with calcifications and cystic changes. A 60-year-old female with epigastric pain. CECT axial (a, b) images demonstrate a complex cystic mass with thin peripheral and coarse central calcifications involving the body of the pancreas (arrows). Intraoperative photograph (c) demonstrates a yellow mass with smooth serosal surface (arrows). Photograph of the bivalved specimen (d) demonstrates a neuroendocrine neoplasm with internal yellow, mucoid-like material and an irregular inner surface. Histological sections (e–h), (H&E, 10x, 40x) show a neuroendocrine neoplasm with extensive fibrosis, calcifications (f) (arrow), and cystic changes. Chromogranin (i) and synaptophysin (j) immunostains were positive (immunostains, 40x, 60x)
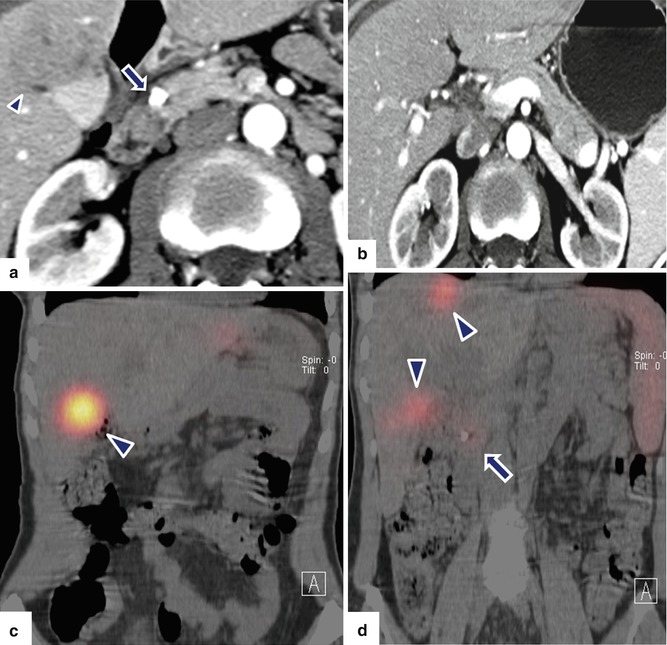
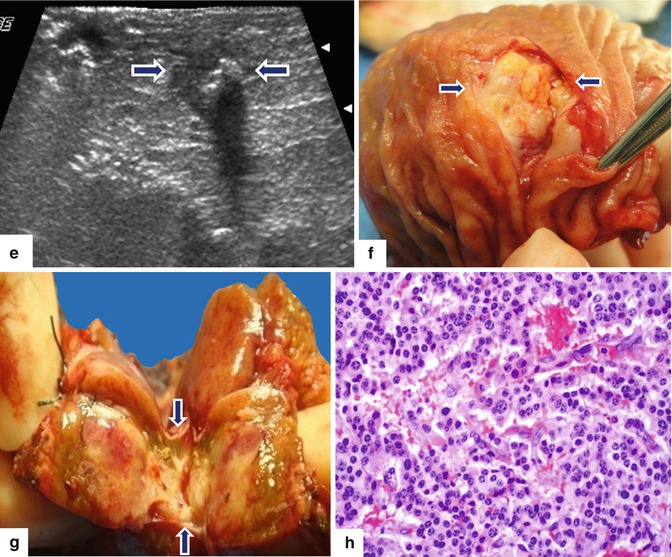
Fig. 10.34
Small, malignant, calcified PNEN with liver metastasis on CT. A 55-year-old female with history of epigastric pain. Upper GI endoscopy showed a duodenal ulcer. Results of ulcer biopsy were negative for malignancy. CECT axial (a, b) images show a coarse calcification in the pancreatic head (arrow). Note a normal pancreatic duct in the body and tail of the pancreas and the presence of a hepatic mass of heterogeneous attenuation in the right lobe (a) (arrowhead). PET/CT coronal (c, d) images show three sites of focus with intense increased uptake of FDG in the right lobe of the liver (arrowheads) and a focus of subtle increased uptake in the head of the pancreas (arrow) around the pancreatic calcification. IOUS transverse (e) image shows a well-defined, small, hypoechoic mass with a coarse calcification in the pancreatic head (arrows). The patient underwent a pancreaticoduodenectomy and a right lobe hepatectomy. Photographs of the duodenum and pancreas (f, g) show a fleshy, yellow-white mass with calcifications (arrows). Histological section (h) (H&E, 60×) shows a neuroendocrine neoplasm, grade 1, composed of uniform cells with nuclei displaying a “salt-and-pepper” chromatin pattern
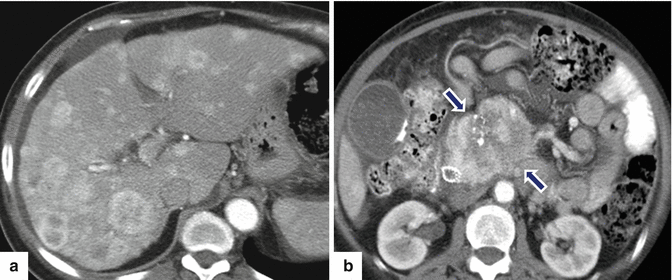
Fig. 10.35
Malignant, calcified nonfunctional PNEN on CT. A 55-year-old female with epigastric pain and weight loss. CECT axial (a, b) images reveal multiple hypervascular hepatic metastases with central necrosis and a large, hypervascular, heterogeneous mass with coarse calcifications in the pancreatic head (arrows). Patient underwent a percutaneous biopsy of the pancreatic mass. Final pathology: neuroendocrine neoplasm, grade 2
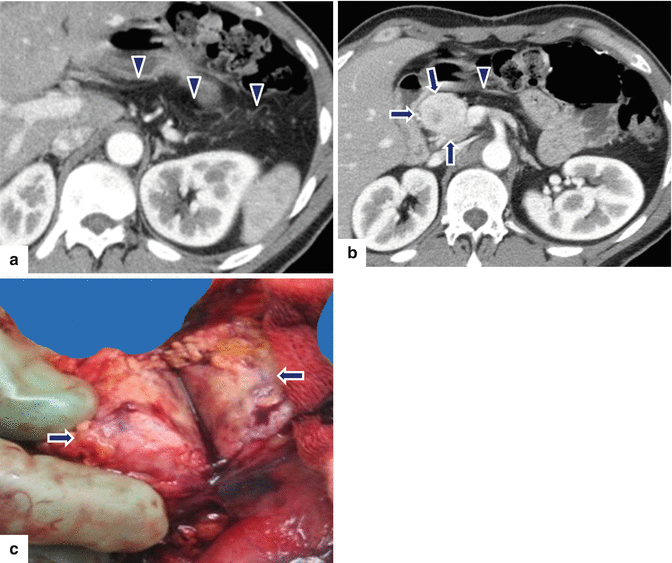
Fig. 10.36
Nonfunctional PNEN associated with pancreatic lipodystrophy. A 45-year-old female patient with an incidental pancreatic finding on chest CT. CECT axial (a, b) images demonstrate an ovoid heterogeneous, hypervascular mass in the head and neck of the pancreas (b) (arrows) associated with complete fatty replacement (a) (arrowheads) of the body and tail of the pancreas. Patient underwent a pancreaticoduodenectomy. Photograph of the bivalved specimen (c) shows a solid, partially hemorrhagic mass in the pancreas (arrows)
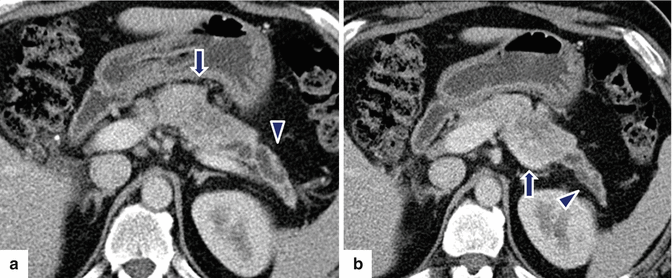
Fig. 10.37
Nonfunctional PNEN associated with obstruction of the pancreatic duct on CT. A 45-year-old male with an incidental pancreatic mass found while undergoing a cholecystectomy. CECT axial (a, b) images display a vascular mass of heterogeneous attenuation involving the pancreatic body (arrows). Note the dilatation of the pancreatic duct distal to this mass (arrowheads) and the atrophy of the pancreatic parenchyma. The patient underwent a distal pancreatectomy and splenectomy. Final pathology: well-differentiated neuroendocrine neoplasm, 5.1 cm in diameter, grade 2
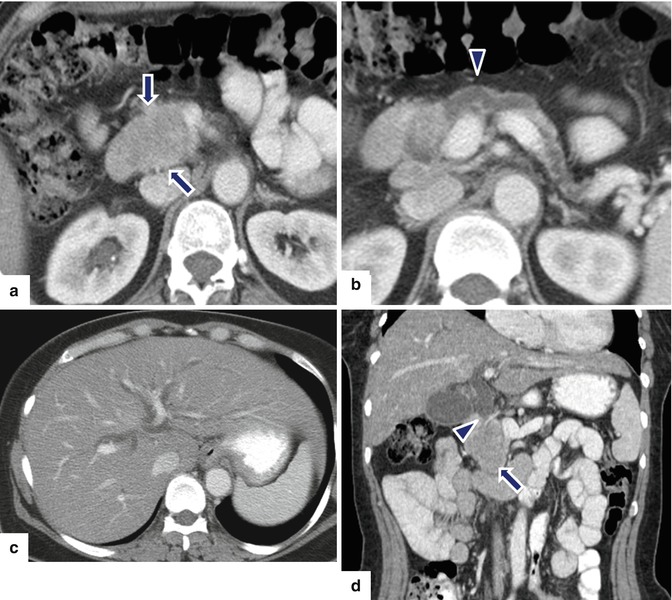
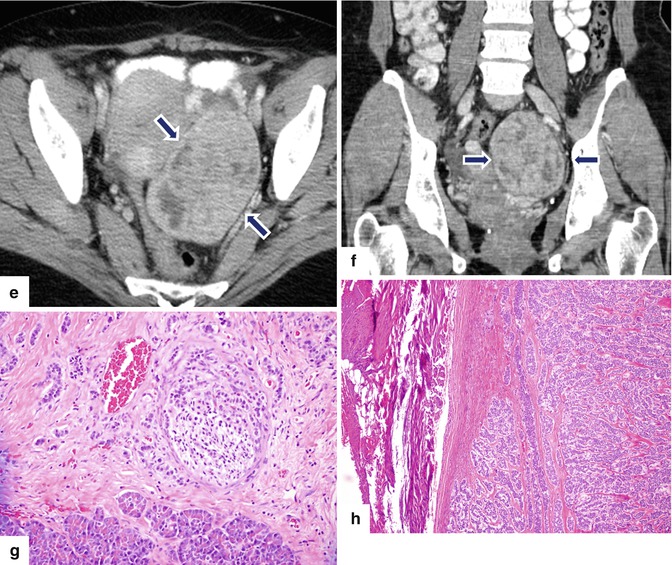
Fig. 10.38
Malignant, nonfunctional PNEN associated with obstruction of the biliary system and pancreatic duct and pelvic metastases on CT. A 51-year-old female with acute onset of painless jaundice. CECT axial (a–c) and coronal (d) images reveal a well-defined mass with heterogeneous attenuation involving the pancreatic head (arrows) associated with obstruction of the pancreatic duct (arrowhead) and dilatation of the intrahepatic biliary system secondary to the obstruction of the common bile duct (arrowhead). CECT axial (e) and coronal (f) images of the pelvis reveal a hypervascular and heterogeneous attenuation on the left side of the pelvic mass (arrows). Patient underwent a pancreaticoduodenectomy and resection of the pelvic mass. Microscopic examination (g) (H&E, 10x) showed a well-differentiated neuroendocrine carcinoma. Metastatic neuroendocrine carcinoma to the ovary (h) (H&E, 10x)
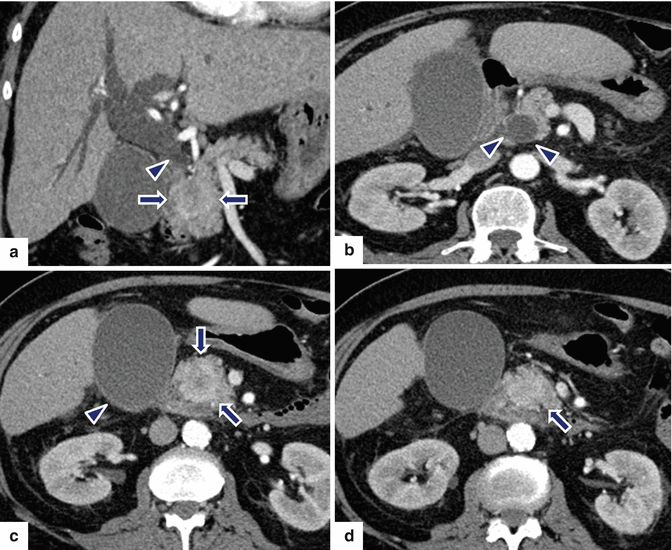
Fig. 10.39
Malignant, nonfunctional PNEN associated with obstruction of the biliary system on CT. A 72-year-old male with history of jaundice, weight loss, and vomiting. CECT coronal (a) and axial (b–d) images demonstrate dilatation of the intra- and extrahepatic biliary system (a, b) (arrowheads) and gallbladder (c) secondary to an ill-defined hypervascular mass involving the head of the pancreas (c, d) (arrows). Note the extension of this mass into the peripancreatic fatty tissues, atrophy of the body and tail of the pancreas, and the absence of dilatation of the pancreatic duct. Patient underwent a Whipple procedure. Final pathology: poorly differentiated neuroendocrine carcinoma, large cell type, with lymphovascular invasion and extension into the peripancreatic soft tissues
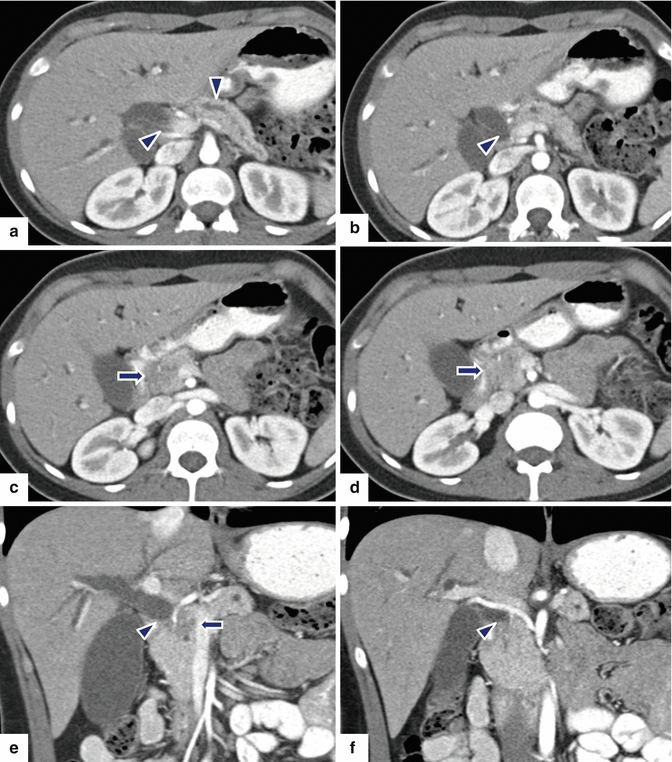
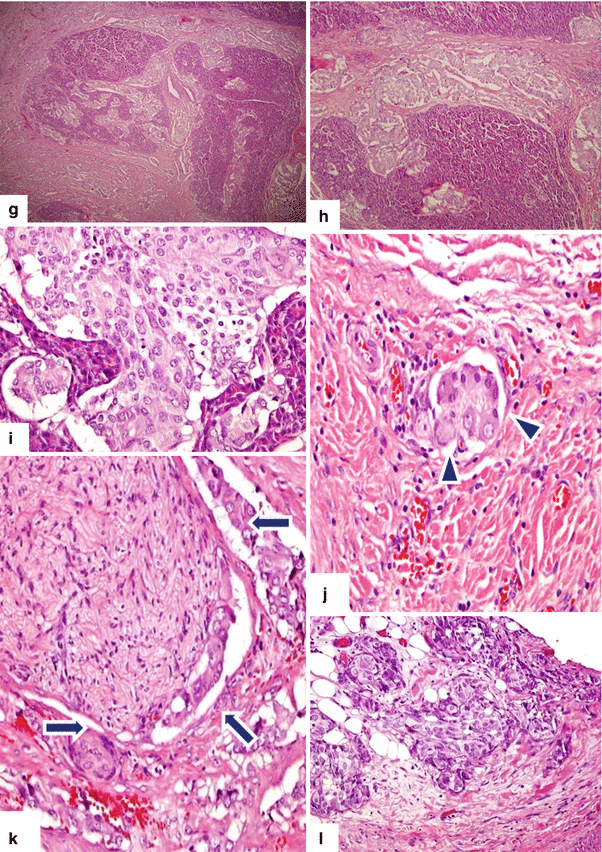
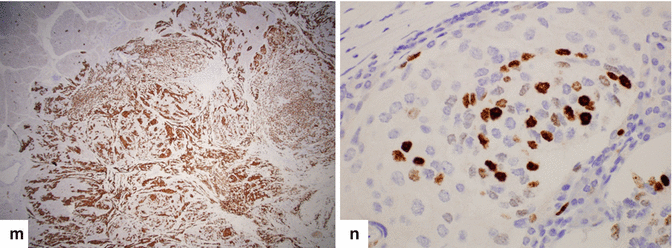
Fig. 10.40
Nonfunctional PNEN associated with obstruction of the biliary system and pancreatic duct on CT. A 20-year-old female patient with history of acute onset of painless jaundice and pruritus. CECT axial (a–d) and coronal (e, f) images show an ill-defined mass with heterogeneous attenuation involving the head of the pancreas (c–e) (arrows) and encasing the gastroduodenal artery, causing obstruction of the common bile duct (a, b, e, f) (arrowheads) and pancreatic duct (a) (arrowhead). The patient underwent a pancreaticoduodenectomy. Histological sections show a poorly differentiated neuroendocrine carcinoma, grade 3 (g) (H&E, 4x), with metastasis to multiple lymph nodes (h, i) (H&E 10x, 60x). Additionally, lymphovascular (j) (arrowheads), perineural (k) (arrows), and adipose tissue (l) invasion was present, (H&E, 60x, 40x)Fig. 10.40 (continued) Immunohistochemistry of the tumor cells reveals strong positivity for synaptophysin (m) and a Ki-67 proliferative rate > 20 %, grade 3 (n)
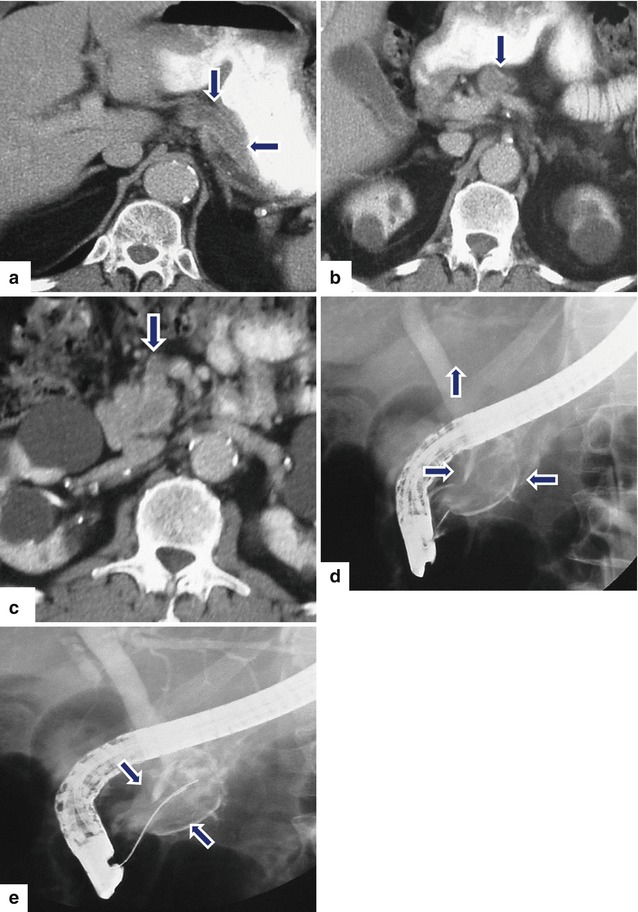
Fig. 10.41
Nonfunctional intraductal PNEN invading the pancreatic duct on CT and ERCP. A 77-year-old male with history of weight loss. CECT axial (a–c) images reveal severe dilatation of the entire pancreatic duct (arrows) up to the level of the major ampulla associated with atrophy of the entire pancreas. ERCP (d, e) images reveal a large filling defect within a dilated pancreatic duct (arrows). Initially, the patient was to undergo a pancreaticoduodenectomy. However, due to the positive surgical margins, a total pancreatectomy and splenectomy was performed
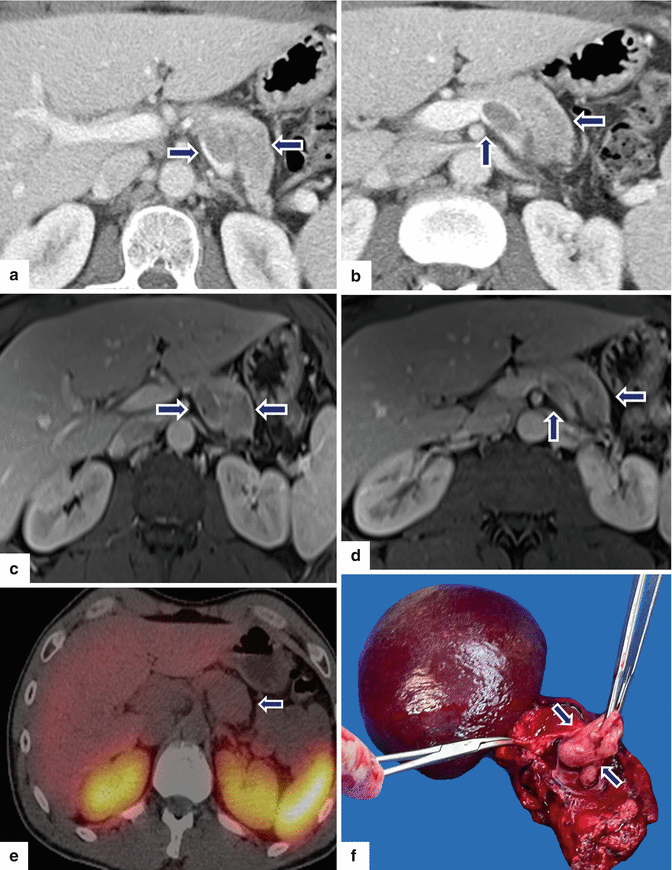
Fig. 10.42
Nonfunctional PNEN associated with vascular invasion on CT and MR. A 55-year-old male with epigastric pain. CECT axial (a, b) images show a low-attenuation mass in the pancreatic body extending into the splenic vein (arrows). Contrast-enhanced fat-suppressed gradient echo T1-weighted axial (c, d) images confirm the presence of a pancreatic mass with heterogeneous signal intensity extending into the splenic vein. Octreotide scan axial (e) image reveals poor radiotracer uptake by this pancreatic mass. The patient underwent a distal pancreatectomy and splenectomy. Photograph of the surgical specimen (f) reveals a white-tan, soft, lobulated, pancreatic mass invading the splenic vein (arrows)
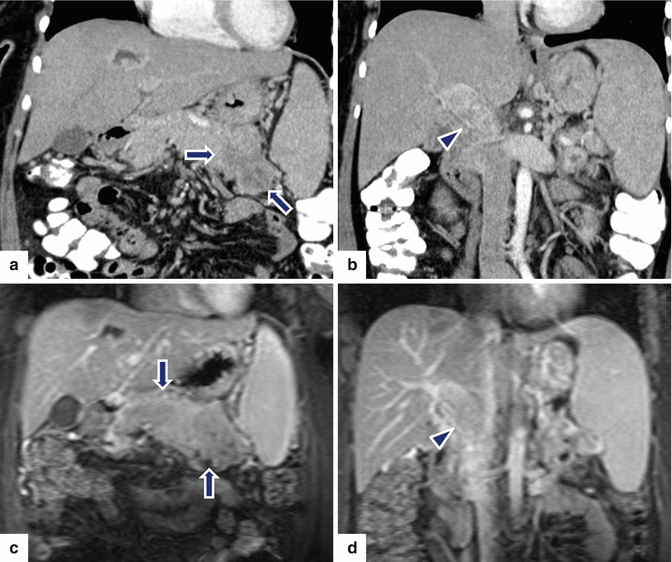
Fig. 10.43
Nonfunctional PNEN associated with vascular invasion on CT and MR. A 35-year-old female with abdominal pain and weight loss. CECT coronal (a, b) images demonstrate an amorphous, low-attenuation mass involving the body and tail of the pancreas (arrows) associated with a thrombus of heterogeneous density in the portal vein (arrowheads). On contrast-enhanced fat suppressed gradient echo T1-weighted coronal (c, d) images, the mass involving the body and tail of the pancreas appears more conspicuous (arrows) and the tumor thrombus in the portal vein was again demonstrated (arrowheads)
Contrast-Enhanced Computed Tomography (CECT) Protocol
Arterial phase images obtained at 20–25 s after the injection of nonionic intravenous contrast
Portal venous phase at 55–50 s after injection
Findings
Well-circumscribed margins, smooth or lobulated
Tumor enhancement:
Small lesion: homogeneous enhancement
Large lesion: heterogeneous enhancement (cystic degeneration, necrosis, fibrosis)
Calcifications (punctuate, coarse)
Signs of Malignancy
Large size
Poorly defined margins
Arterial vascular encasement (gastroduodenal, hepatic, splenic arteries)
Venous invasion: (splenic vein, portal vein)
Enlarged peripancreatic nodes: hypervascular, homogeneous or heterogeneous
Hepatic metastases: hypervascular, homogeneous or heterogeneous, or peripheral rim enhancing
Biliary obstruction
Practical Pearls
Pancreatic duct dilatation associated with parenchymal atrophy and/or pancreatic lipodystrophy distal to a PNEN is rarely seen. These findings may be associated with either benign or malignant tumors.
Intraductal PNENs without a pancreatic parenchymal lesion may also occur but are exceptionally rare (Fig. 10.41).
10.4.4.3 Magnetic Resonance Imaging (MRI) (Figs. 10.44–10.54)
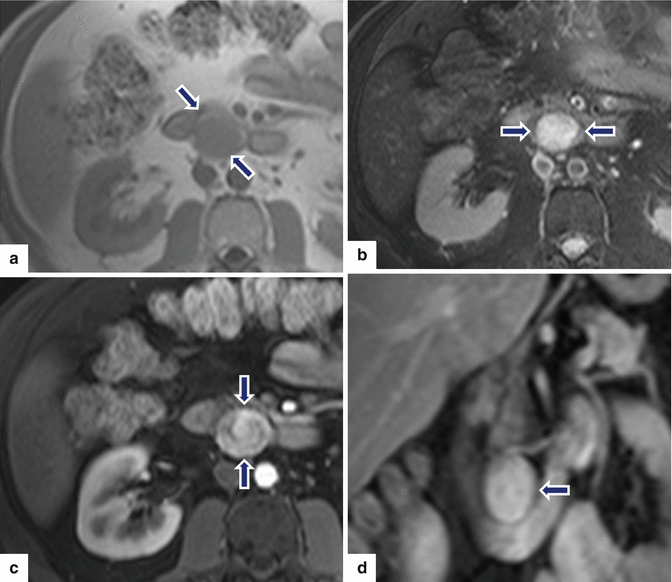
Fig. 10.44
Nonfunctional PNEN on MR. A 62-year-old female with vague abdominal pain. T1W axial (a) image shows an exophytic, round, low signal intensity mass arising from the pancreatic head (arrows). On fat-suppressed T2W axial (b) image, this mass shows a high signal intensity (arrows). On contrast-enhanced fat-suppressed gradient echo axial (c) and coronal images (d), this mass shows a heterogeneous, vascular enhancement (arrows)
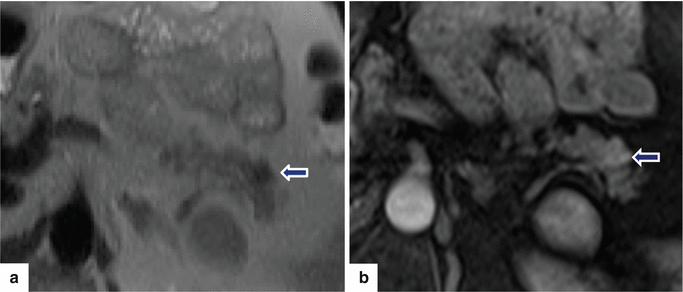
Fig. 10.45
Small, nonfunctional PNEN on MR. A 45-year-old male with an incidental finding. T1W axial (a) image demonstrates a small, lobulated, low signal intensity mass in the tail of the pancreas (arrow). Contrast-enhanced fat-suppressed gradient echo T1 weighted axial (b) image shows a heterogeneous, vascular enhancement by this mass (arrow)
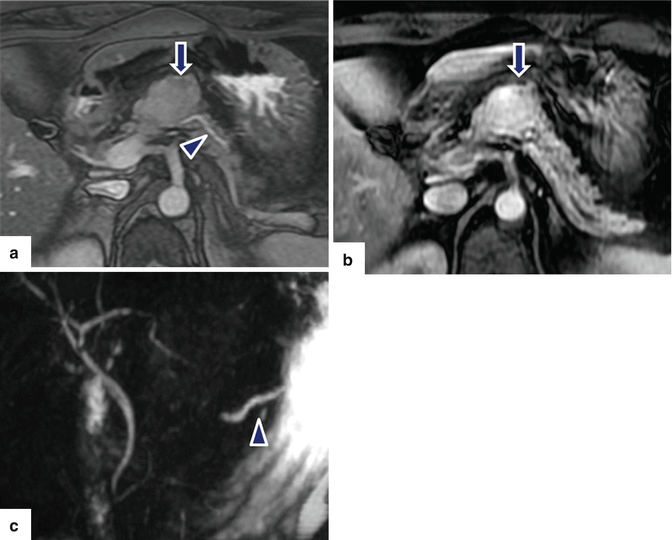
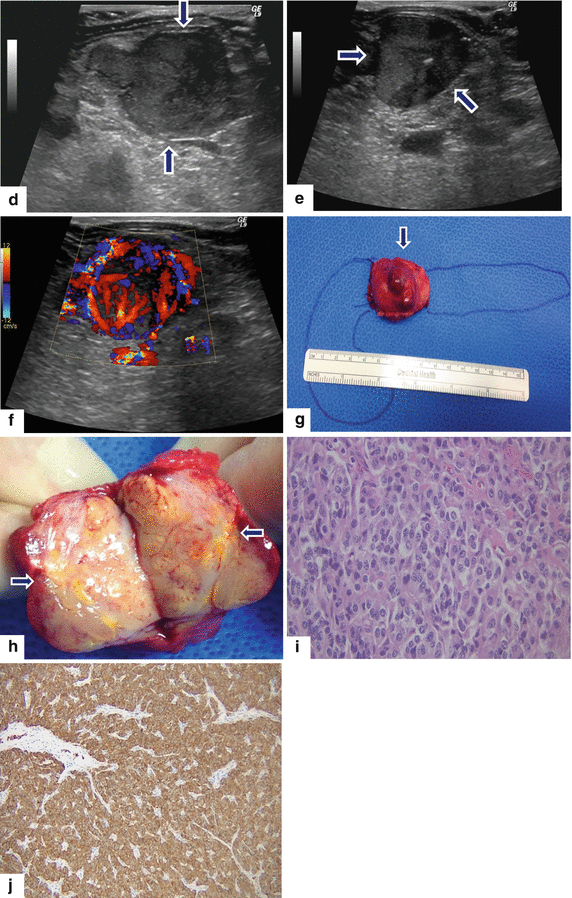
Fig. 10.46




Nonfunctional PNEN associated with obstruction of the pancreatic duct on MR. A 48-year-old female with history of abnormal liver function tests in which an abdominal MR was performed. Fat-suppressed T2W axial (a) image shows an ovoid, low signal intensity mass involving the pancreatic body (arrow) associated with mild dilatation of the distal pancreatic duct (arrowhead). Contrast-enhanced fat-suppressed gradient echo T1 weighted axial (b) image shows intense, homogeneous, contrast enhancement by this mass (arrow). MRCP thick slab (c) image shows abrupt obstruction of the pancreatic duct distal to the pancreatic mass (arrowhead). IOUS gray scale (d, e) and color Doppler transverse (f) images show a lobulated hypoechoic, hypervascular mass (d, e) (arrows) with a coarse calcification. The patient underwent a central pancreatectomy. Photograph of the surgical specimen (g) shows a bilobulated pancreatic mass (arrow). Photograph of the bivalved gross specimen (h) shows a fleshy, tan, round, irregular mass with prominent white bands of fibrosis, residual yellow pancreatic tissue, and scattered, small hemorrhagic foci (arrows). Histological sections (i) show round cells with a “salt-and-pepper” appearance of the chromatin (H&E, 40×). Chromogranin immunostain (j) is strongly positive (immunohistochemistry, 10×)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree