Chapter Outline
Classification of Pancreatitis
Pancreatitis is one of the most complex and clinically challenging of all abdominal disorders. It remains a major diagnostic challenge because its clinical manifestations are as protean as its causes. Indeed, only one in five severe cases of acute pancreatitis is recognized to be severe at initial presentation, and 42% of fatal cases of acute pancreatitis do not have a correct diagnosis before autopsy.
Since the late 1970s, cross-sectional imaging and, in particular, high-resolution, bolus contrast–enhanced multidetector helical computed tomography (CT) have dramatically improved the diagnosis and treatment of acute pancreatitis. More recently, magnetic resonance imaging (MRI) has shown that it can have a role in imaging of patients with pancreatitis. The focus of this chapter is on the contribution of radiology to the assessment and management of patients with acute or chronic pancreatitis.
Classification of Pancreatitis
Pancreatitis is classified according to clinical, morphologic, and histologic criteria on the basis of the Marseille and Cambridge symposia and the 1992 International Symposium in Atlanta, Georgia, and more recently the Acute Pancreatitis Classification Working Group in 2008.
Acute Pancreatitis
Etiology
Although the causes of pancreatitis are diverse, alcoholism and biliary tract disease (gallstones) account for approximately 90% of cases in the United States. The relative frequency of these causes varies with the country and population of patients examined. Alcoholic pancreatitis is more common in urban and Veterans Administration hospitals, whereas gallstone pancreatitis predominates in suburban and rural hospitals. The incidence of acute pancreatitis is 0.005% to 0.01% in the general population. Approximately 10% to 30% of patients with acute pancreatitis will never have the cause of the disorder established and are given the diagnosis of “idiopathic” pancreatitis. Biliary sludge and microlithiasis are probably responsible for the development of pancreatitis in the majority of these cases.
Identification of the cause of pancreatitis is important because it helps determine therapy. It may limit further unnecessary examinations and may improve the patient’s long-term outcome.
Pathophysiology
The precise mechanisms of pathogenesis of acute pancreatitis are not completely understood. Alcohol increases the risk for pancreatitis through multiple mechanisms. More than 95% of heavy alcohol users, however, never develop significant pancreatitis, which suggests that this is a complex syndrome, with other risk factors involved. Hypotheses and theories to explain alcoholic pancreatitis include necrosis-fibrosis sequence, duct obstruction, leakage of enzymes from the pancreatic duct, abnormal synthesis of digestive enzymes, toxic metabolites, altered pancreatic blood flow, mitochondrial damage, and activation of pancreatic stellate cells, which produce collagen in the fibrotic pancreas.
In alcoholic pancreatitis, the toxic effect and chemical alterations of the exocrine secretions produced by alcohol lead to protein precipitates that obstruct the pancreatic ducts. In addition, alcohol can lead to duodenitis, edema, and spasm of the papilla of Vater, further contributing to ductal obstruction.
Alcohol also indirectly stimulates pancreatic secretion by inducing an increase in gastrin and secretin levels and decreases the level of zymogen inhibitor, resulting in the premature activation of trypsin. In acute pancreatitis, these activated pancreatic enzymes are extravasated, causing pancreatic autodigestion and necrosis. In cholelithiasis-induced pancreatitis, there is obstruction of the common biliopancreatic channel by a stone (Opie stone), with resultant reflux of bile into the pancreatic duct and activation of pancreatic enzymes. Gallstones are found in the stool of most of these patients. This is the rationale for emergency cholecystectomy or endoscopic retrograde cholangiopancreatography (ERCP)–directed sphincterotomies in the treatment of gallstone pancreatitis. Reflux of contrast material into the pancreatic duct during intraoperative cholangiography is much more common in patients with gallstone pancreatitis than in patients undergoing cholecystectomy who do not have pancreatitis.
Pancreas divisum, discussed in detail in Chapter 96 , is also postulated to induce this disorder by a functional obstruction at the accessory papilla of Santorini. A variety of other causes, such as drugs, calcium, endotoxins, hyperlipidemia, viral infections, ischemia, anoxia, and trauma, can also activate proteolytic enzymes in the pancreas that lead to autodigestion and stimulate other enzymes, such as bradykinin and vasoactive substances, that cause vasodilation, increased vascular permeability, and edema.
Leukocytes attracted by the pancreatic injury release inflammatory mediators called cytokines, which have an important role in disease progression and multisystem complications of acute pancreatitis. Biologically active compounds, such as phospholipase A 2 , tumor necrosis factor, polymorphonuclear cell elastase, complement factor, interleukins, and leukotrienes, are released into the systemic circulation, stimulating the production of other mediators and leading to distant organ failure. Some of these mediators, such as tumor necrosis factor, are also toxic to acinar cells and may contribute to pancreatic injury and necrosis. These inflammatory products occur early in the course of the disease and can be used as indicators of severity and prognosis in acute pancreatitis.
Pancreatitis has been shown to have an increased prevalence in patients with acquired immunodeficiency syndrome (AIDS), explained either by the effects of medication (pentamidine and 2′,3′-dideoxyinosine [DDI]) or by secondary opportunistic infections.
No dependable correlation exists between the etiology and the severity of disease. In general, most cases of necrotizing pancreatitis are associated with alcoholism and hyperlipidemia, whereas severe pancreatitis is less commonly noted with other causes.
There are several major pathologic categories of acute pancreatitis. Acute interstitial edematous pancreatitis is the mildest form of pancreatitis. It is characterized by absent or minimal pancreatic and systemic dysfunction and a rapid response to medical therapy without complications. Only pancreatic edema and mild cellular infiltrate are present. The gland may enlarge to three times its normal size and become firm. There may be a few small, scattered foci of necrosis and saponification in the peripancreatic fatty tissue. Other organs generally are not involved. Necrotizing pancreatitis is a far more severe form of pancreatitis in which varying degrees of systemic and distal organ failure and potentially lethal complications can occur. Extensive fat necrosis, hemorrhage, and necrotic liquefaction of the pancreatic parenchyma and adjacent peripancreatic tissues and fascial planes are seen. In addition, a variety of intermediary forms of pancreatitis occur in clinical practice. The degree and extent of injury appear to be related to the quantity, rate of production, and activity of the highly lipolytic and proteolytic extravasated enzymes.
Definitions
Considerable ambiguity and overlap of pathologic features exist in describing the complications of pancreatitis. The following are definitions of commonly encountered sequelae that are discussed in subsequent sections.
Acute pancreatitis is diagnosed when two of the three following criteria are present: (1) abdominal pain related to acute pancreatitis, (2) serum amylase or lipase activity greater than three times the normal level, and (3) imaging findings characteristic of acute pancreatitis.
According to the revised Atlanta classification of acute pancreatitis, acute pancreatitis can be divided into two major types: interstitial edematous pancreatitis and necrotizing pancreatitis.
Interstitial Edematous Pancreatitis.
Most patients have diffuse (or less commonly focal) pancreatic enlargement from interstitial edema. The pancreas enhances relatively homogeneously without necrosis, and there is peripancreatic inflammation with mild stranding or fluid. The clinical findings generally resolve within the first week. When imaging is performed within the first days after onset of pancreatitis, it may be difficult to distinguish interstitial edematous pancreatitis from patchy necrosis. Follow-up contrast-enhanced CT 3 to 7 days later may be helpful in the distinction.
Acute Peripancreatic Fluid Collection.
Fluid collection in peripancreatic regions typically occurs within 4 weeks of symptom onset, mostly after interstitial edematous pancreatitis. It lacks nonliquefied material and a definable wall.
Necrotizing Pancreatitis.
On imaging, necrotizing pancreatitis is defined by lack of enhancement of the pancreatic tissue. Necrosis most commonly involves the pancreas and peripancreatic tissues, followed by the pancreas and, least commonly, the peripancreatic tissues. Necrotizing pancreatitis is quantified on the basis of studies by Balthazar as less than 30%, 30% to 50%, and more than 50%. More recently, it has been graded by modified CT as less than 30% or more than 30%.
Acute Necrotic Collection.
Acute necrotic collection refers to a persistent collection containing fluid and necrotic material of various amounts associated with necrotizing pancreatitis. This collection accumulates in the pancreas or peripancreatic tissue and occurs within 4 weeks after development of necrotizing pancreatitis.
Pancreatic Pseudocyst.
Pancreatic pseudocysts are localized collections of pancreatic fluid confined by a capsule of fibrous or granulation tissue; they typically develop during 4 weeks after an acute episode or can be associated with stigmata of chronic pancreatitis. Pseudocysts lack a true epithelial lining, which distinguishes this entity from a cyst or cystic neoplasm. They are manifestations of a local complication of acute non-necrotic pancreatitis and should not contain solid material. Current literature estimates that 50% of asymptomatic pseudocysts smaller than 6 cm resolve spontaneously. Pseudocysts larger than 7 cm in diameter are likely to require intervention, particularly in patients with alcoholic pancreatitis. Persistent pseudocysts often communicate with the pancreatic duct and have the potential to rupture, to bleed, to become infected, or to cause a pseudoaneurysm. Infected pseudocysts require drainage.
Walled-off Necrosis.
Walled-off necrosis is a persistent collection of necrotic tissue with a thickened wall that lacks a true epithelial lining; it arises in the setting of necrotizing pancreatitis. This collection accumulates in the pancreas or peripancreatic tissue usually 4 weeks or more after onset of necrotizing pancreatitis and may or may not be infected. Unlike a pseudocyst, walled-off necrosis contains necrotic pancreatic parenchyma or fat. The liquefied components may be better characterized by MR or ultrasound, including endoscopic ultrasound (EUS), to guide drainage if it is required.
Phlegmon.
Phlegmon is a term formerly used to imply the presence of a heterogeneous masslike enlargement of the pancreas and retroperitoneal tissues. It does not correlate with the presence of infection or necrosis. Because the term has different meanings to gastroenterologists, internists, and surgeons, it should be avoided according to the revised Atlanta criteria.
Abscess.
Pancreatic abscess develops from infected, extravasated pancreatic secretions. It usually occurs 3 weeks or more after the initial attack or may develop as a secondary infection of a pseudocyst. This term has fallen out of favor with the revised Atlanta classification system, and the term infected pseudocyst is used instead.
Pseudoaneurysm.
A pseudoaneurysm is a focal area of dilation of a splanchnic artery that may occur as a result of inflammatory weakening of the arterial wall by enzymes liberated in acute pancreatitis. Pseudoaneurysms usually are found in the splenic, gastroduodenal, and pancreaticoduodenal arteries and can be freestanding or associated with a pseudocyst. Intermittent or life-threatening hemorrhage into a pseudocyst, retroperitoneum, or peritoneal cavity may occur.
Clinical Findings
The clinical diagnosis of pancreatitis is often difficult. Patients’ symptoms range from mild abdominal pain, nausea, vomiting, fever, tachycardia, and abdominal distention to severe abdominal pain and shock. Most patients have abdominal tenderness and guarding. These findings are nonspecific, and the differential diagnosis usually includes acute cholecystitis, bowel obstruction or infarction, perforated viscus, renal colic, duodenal diverticulitis, aortic dissection, appendicitis, and ruptured abdominal aortic aneurysm. In very severe cases, flank ecchymosis (Grey Turner’s sign) or periumbilical hematoma (Cullen’s sign) may be present.
Consequently, a battery of laboratory tests has been developed to diagnose and to grade pancreatitis. These tests include evaluation of serum amylase, lipase, serum/urinary amylase ratio, pancreatic isoamylase, immunoreactive trypsin, chymotrypsin, elastase, serum cyclic adenosine monophosphate, C-reactive protein, urinary trypsinogen-2, and methemalbumin. Serum amylase and lipase levels are the most commonly used measures to diagnose pancreatitis. Unfortunately, these values are elevated in only 80% to 90% of patients with acute pancreatitis. Amylase is rapidly secreted by the kidneys and may return to normal levels during the first 48 to 72 hours. Pancreatitis caused by gallstones, microlithiasis, or drugs is often associated with a greater elevation in amylase than in lipase. The amylase level relative to lipase tends to be lower in alcoholic pancreatitis, hypertriglyceridemia-induced pancreatitis, neoplasia, and chronic pancreatitis. There is no correlation between the levels of serum amylase and the severity of acute pancreatitis; patients with mild forms of disease may exhibit levels above 1000 units, whereas patients with severe necrotizing pancreatitis may have normal or low amylase levels. Furthermore, hyperamylasemia may be seen in other acute abdominal conditions, such as bowel obstruction, bowel infarction, gangrenous cholecystitis, and perforated ulcer. Elevated lipase is more specific for pancreatitis, but the level does not predict the etiology.
The onset of acute pancreatitis is defined from the time of onset of abdominal pain and not date of hospital admission. The clinical course of acute pancreatitis varies from mild and self-limited disease to shock, overwhelming sepsis, and death. The revised Atlanta classification divides acute pancreatitis into two broad categories: nonsevere acute interstitial edematous pancreatitis; and severe acute pancreatitis, which often involves necrosis of the gland or peripancreatic tissues. Characterization of acute pancreatitis can be considered on the basis of early and late phases of the disease. The early phase of acute pancreatitis appears within the first week and shows systemic reaction of the body to pancreatic enzymatic insults and injuries. This phase usually resolves within a week. The clinical features during this period are commonly the signs of systemic inflammatory response syndrome. The late phase of acute pancreatitis is seen only in moderately severe or severe acute pancreatitis. During this phase, local complications of acute pancreatitis and continuation of systemic signs of inflammation are noted; therefore, both clinical and imaging criteria are important.
Mild acute pancreatitis usually improves within the first week and during the early phase of disease. There is no evidence of organ failure or local or systemic complications of acute pancreatitis, and an imaging examination is often not needed in mild cases. Moderately severe acute pancreatitis is defined by the presence of local or systemic complications of acute pancreatitis or the existence of transient organ failure. By definition, transient organ failure in a patient suffering from acute pancreatitis lasts less than 48 hours. Severe acute pancreatitis determines when organ failure is evident for more than 48 hours.
Radiologic Findings
Cross-sectional imaging has made a significant contribution to the diagnosis and staging of acute pancreatitis. Radiologic imaging of patients with suspected pancreatitis has five major objectives: (1) to exclude other abdominal disorders that can mimic acute pancreatitis; (2) to confirm the clinical diagnosis of acute pancreatitis; (3) to evaluate the extent and nature of pancreatic injury and peripancreatic inflammation in an attempt to stage the severity of disease; (4) to determine the etiology of acute pancreatitis; and (5) to serve as a guide for therapeutic intervention and response to therapy. Although plain films and contrast studies have been used in the past for the diagnosis of pancreatitis, they have been largely replaced by cross-sectional studies, which have significantly greater diagnostic accuracy. Findings and signs indicative of pancreatitis on different imaging modalities are briefly discussed here, with an emphasis on CT and MRI.
Abdominal Plain Films
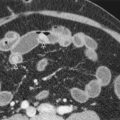
Abnormalities in the abdominal gas pattern are the most frequent findings on abdominal plain films and range from a gasless abdomen to an ileus pattern. The most common findings are a small bowel ileus (42%), in which an adynamic sentinel loop is seen, and the colon cutoff sign (56%), related to spasm of the splenic flexure with distal paucity of gas caused by spread of the pancreatic inflammation into the phrenocolic ligament. Although it was originally described in abdominal plain films, the colon cutoff sign can also be seen on CT ( Fig. 97-1 ).

Chest Films
Approximately one third of patients with acute pancreatitis demonstrate pulmonary and chest abnormalities: elevated diaphragms, pleural effusions, basal atelectasis, pulmonary infiltrates, and the adult respiratory distress syndrome. Abnormalities on chest films can be useful to determine the severity of acute pancreatitis.
Barium Studies
Meyers and Evans have emphasized the importance of the ligaments and mesenteries of the subperitoneal space ( Fig. 97-2 ) in the spread of pancreatitis to understanding of the radiographic findings. Extravasated pancreatic enzymes commonly enter the lesser sac and spread to the transverse mesocolon, phrenocolic ligament, and small bowel mesentery, causing serosal inflammation and irritation of the gastrointestinal tract. Thickening and spiculation of mucosal folds in the stomach and duodenum can be seen on CT and barium studies. Varying degrees of atony associated with spastic segments of the duodenum, jejunum, and transverse colon can also be present.

Cholangiography
ERCP is generally not performed during an acute episode of pancreatitis because it may exacerbate the inflammation or introduce an infection. The pancreatic duct is usually normal but can be compressed. Some gastroenterologists advocate ERCP with sphincterotomy to alter the course of pancreatitis when it appears that multiple stones are passing through the common bile duct. ERCP can also demonstrate other causes of pancreatitis, such as pancreas divisum, choledochocele, choledochal cyst, perivaterian duodenal diverticulum, pancreatic or bile duct carcinoma, and ampullary carcinoma (see Chapter 74 ). Magnetic resonance cholangiopancreatography (MRCP) is often preferred to investigate the cause of pancreatitis, however, avoiding the complications associated with ERCP.
Angiography
Angiography is usually not performed in patients with acute pancreatitis unless the presence of a pseudoaneurysm that could be treated with transcatheter embolization is suspected. It can also be helpful to elucidate vascular causes of pancreatitis, such as vasculitis, polyarteritis nodosum, post–aortic aneurysm resection, after transplantation, Ortner’s syndrome, systemic lupus erythematosus, low-flow states, shock, and diabetes.
Ultrasonography
Abnormal findings are seen at sonography in 33% to 90% of patients with acute pancreatitis. The classic sonographic appearance in pancreatitis is a diffusely enlarged hypoechoic pancreas. Less commonly, focal enlargement is present. The echogenicity of the pancreas in acute pancreatitis is extremely variable and depends on a number of factors: the timing of the sonographic study, with maximal decrease in echogenicity occurring 2 to 5 days after the initial episode of acute abdominal pain; the amount of intrapancreatic fat (with age, the pancreas is replaced by fat and hence becomes more echogenic); the presence of hemorrhage; the presence of underlying chronic pancreatitis with calcification; and the degree of extrapancreatic spread of acute pancreatitis. Echogenicity is also subjective because the usual internal standard used to gauge pancreatic texture is the liver, which often has altered architecture caused by fatty infiltration, cirrhosis, or alcoholic pancreatitis. Similarly, size changes are difficult to assess without a baseline scan because the overall volume of the gland is variable and diminishes with advanced age.
The pancreatic duct may dilate, particularly if the inflammation is confined to the pancreatic head. Focal intrapancreatic masses may be due to an acute fluid collection, hemorrhage, or ill-defined and hypoechoic pancreatic enlargement that may sonographically simulate carcinoma. Cystic masses should be scrutinized with Doppler ultrasound studies to exclude pancreatic pseudoaneurysms. Lesser sac fluid collections are often seen and may produce a “butterfly” appearance.
The foregoing discussion assumes that the pancreas is well visualized. In acute pancreatitis, bowel gas and other factors will limit visualization of the gland in one fourth to one third of patients. This is one of four major limitations of ultrasound in this clinical setting. The second is the inability of ultrasound to completely define the complex extrapancreatic spread of inflammation along fascial planes and within the peripancreatic compartments. It is particularly limited in visualizing spread into the transverse mesocolon. Third, ultrasound cannot specifically reveal areas of pancreatic necrosis in patients with severe pancreatitis, information that provides important insights into the ultimate prognosis of necrotizing pancreatitis. Finally, only CT, MRI, or angiography can diagnose many gastrointestinal and vascular complications of acute pancreatitis.
What, then, is the role of sonography in acute pancreatitis? It is a good screening test in patients with suspected biliary pancreatitis and a mild clinical course. It is helpful to detect gallstones, for which CT is not effective. It is also useful in thin patients with mild edematous pancreatitis that promptly responds to conservative therapy. Ultrasound has limitations in the detection of distal common duct stones. It also has limitations in the diagnosis of necrosis. Ultrasound may be used to assess the presence or absence of solid necrotic material and to differentiate it from cystic material to help guide drainage when it is being considered. CT is preferred for patients with clinically severe pancreatitis; those whose disease fails to respond to conservative therapy; acutely ill patients who pose diagnostic dilemmas; and patients with complications, such as infected pseudocyst, hemorrhage, pseudoaneurysm formation, and pancreatic necrosis.
The use of tissue harmonic imaging improves image quality, delineation of the pancreatic tail, lesion conspicuity, and fluid-solid differentiation relative to conventional B-mode sonography. Tissue harmonic sonography was able to detect abnormalities in 91% of patients with acute pancreatitis. The authors found that the two most useful sonographic findings were extrapancreatic inflammation and parenchymal inhomogeneity. It was difficult, however, to distinguish between fluid collections and extrapancreatic inflammation. Nevertheless, the accuracy of CT is still superior to that of tissue harmonic sonography in the evaluation of acute pancreatitis, as even with tissue harmonics, sonography does not demonstrate necrosis or other complications as well as CT does.
Computed Tomography and Magnetic Resonance Imaging
CT is the premier imaging test in the diagnosis and management of patients with acute pancreatitis. It visualizes the gland, the bowel, the retroperitoneum, the abdominal ligaments, the mesenteries, and the omentum in their entirety. In addition, it may help determine the etiology, stage severity, and detect complications of pancreatitis; it can be used to guide interventional procedures, such as fine-needle aspiration biopsy or catheter placement. However, the radiation dose delivered by CT may be a significant factor for young patients with pancreatitis requiring multiple examinations. MRI may be an option for these patients; however, it is more costly than CT. Other advantages of MRI include its high soft tissue contrast resolution and ability to evaluate the common bile duct, pancreatic duct, and parenchyma in a single examination. Before the awareness of nephrogenic systemic fibrosis, contrast-enhanced MRI could be used in patients with renal insufficiency when contrast-enhanced CT could not be performed. MRI, however, is limited in the more acute setting when the patient is very ill, as patients need to be able to hold the breath and be relatively stable for the longer examination. MRI may be helpful to distinguish peripancreatic fluid collections as solid debris from cystic collections and show the compositions of pseudocysts better than multidetector CT (MDCT). Diffusion-weighted MRI can be helpful in the diagnosis of acute pancreatitis.
CT Technique.
The technique used in evaluating patients with pancreatitis should be individualized. The best diagnostic images are obtained with MDCT by achieving optimal vascular and parenchymal enhancement and avoiding respiratory motion. A rapid 5 mL/s intravenous bolus of 150 mL of 60% nonionic contrast material is injected. We routinely use a two-phase imaging technique. Images in the pancreatic and hepatic venous phases are obtained with 2- to 3-mm slice thickness. In addition, unenhanced images may be helpful through the pancreas. Images can be reconstructed into any plane with various thickness and section intervals at any level in the scanned volume.
MRI Technique.
The role of MRI has become increasingly more important with the advent of high-field-strength imaging, phased-array surface coils, MR-compatible power injectors, rapid gradient-echo breath-hold techniques, and fat suppression. These technologic advances allow faster sequences with high-resolution images that are largely free of artifacts. T1-weighted fat-suppressed sequences before and after gadolinium administration and T2-weighted sequences are essential for pancreatic evaluation. The T1-weighted fat-suppressed sequences are able to detect focal or diffuse abnormalities in the pancreas and to delineate the peripancreatic fat planes. With T1 relaxation time shorter than that of the liver, the normal pancreas has the highest signal intensity of intra-abdominal organs. This is attributed to the abundance of acinar proteins, endoplasmic reticulum, and paramagnetic ions within the pancreas. Contrast-enhanced T1-weighted images in the arterial phase show intense enhancement of the normal pancreas, which becomes less intense on subsequent phases. T2-weighted sequences show fluid as high signal intensity, allowing assessment of the pancreaticobiliary ducts and detection of fluid collections and peripancreatic edema.
Diagnosis.
The imaging findings in acute pancreatitis are similar, regardless of etiology, with the exception of traumatic pancreatitis, in which pancreatic lacerations cause high-density (50-90 HU) hematomas. MRI can be helpful in determining the etiology of pancreatitis, including choledocholithiasis, pancreas divisum, and underlying tumors. In the acute setting, however, the etiology may be difficult to determine because of the significant inflammation, which may obscure stones, pancreaticobiliary ducts, or underlying masses.
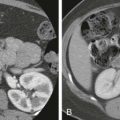
In mild forms of pancreatitis, CT and MRI may show normal findings or a slight to moderate increase in the size of the pancreas. Mild inflammation may surround an otherwise normal-appearing gland ( Fig. 97-3 ). Alternatively, the pancreas may be diffusely enlarged, with shaggy and irregular contour, and slightly heterogeneous parenchyma, which enhances less than normal ( Fig. 97-4 ). On MRI, there may be decreased pancreatic signal intensity on T1-weighted fat-suppressed sequences before and after gadolinium administration, depending on the degree of inflammation and edema ( Fig. 97-5 ).



In more advanced cases, extravasation of pancreatic fluid leads to the formation of intrapancreatic and extrapancreatic fluid collections ( Fig. 97-6 ). Because the pancreas does not have a well-developed fibrous capsule, pancreatic secretions commonly extravasate in the retroperitoneum, most often in the anterior pararenal space ( Fig. 97-7 ; see also Fig. 97-1 ). CT superbly depicts peripancreatic inflammation, fluid, and occasionally mild thickening of the adjacent fascial planes (see Figs. 97-1 and 97-3 ). Peripancreatic inflammatory stranding is also well seen on MRI (see Fig. 97-5 ).


A more unusual segmental form of acute pancreatitis occurs in as many as 18% of patients. The CT findings are similar to those in diffuse pancreatitis; however, only part of the gland is involved either exclusively or predominantly. The segment most often involved is the pancreatic head. This form of pancreatitis is usually mild and is most often associated with stone disease. However, patients with segmental pancreatitis should be carefully evaluated to exclude the less common possibility of adenocarcinoma simulating pancreatitis or a mass leading to pancreatitis ( Figs. 97-8 and 97-9 ). It may be appropriate to further investigate these patients with EUS, MR, ERCP, or biopsy or to perform short-term follow-up CT or MRI, especially if they are older or do not have risk factors for pancreatitis.


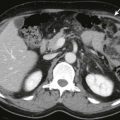
In severe forms of necrotizing pancreatitis, the gland may become enlarged, and it is commonly enveloped by high-attenuation heterogeneous fluid collections. Because of high-attenuation exudates, the presence of pancreatic necrosis cannot be assessed on CT unless the gland is imaged during the late arterial–early portal venous phase of a rapid bolus intravenous injection of contrast medium ( Fig. 97-10 ). On MRI, peripancreatic high signal intensity may be seen on T1-weighted fat-suppressed sequences related to fat necrosis and hemorrhage ( Fig. 97-11 ). This finding has been associated with a worse prognosis. Patchy areas of absence of enhancement, fragmentation, and liquefaction necrosis can be detected on CT or MRI ( Fig. 97-12 ). Poorly defined peripancreatic exudates obliterate the peripancreatic fat, envelop the pancreas, dissect fascial planes, and penetrate through fascial and peritoneal boundaries and ligaments. These collections most often occur in the anterior pararenal space around the body and tail of the pancreas and within the lesser peritoneal sac ( Fig. 97-13 ). Penetration through Gerota’s fascia with involvement of the perirenal fat and kidneys occurs rarely. When fluid collections are massive, they tend to extend inferiorly along the pararenal spaces in the left flank or bilaterally. They can course over the psoas muscle, enter the pelvis (see Fig. 97-13B ), and sometimes extend into the groin. Exudates can invade the small bowel mesentery, the transverse mesocolon, and the posterior pararenal spaces (see Fig. 97-7 ). Fluid can penetrate into solid organs, such as the spleen or the liver, and even the mediastinum. Splenic involvement is most common because of the close anatomic relationship of the pancreatic tail and splenic hilum. Subcapsular or intrasplenic fluid collections or pseudocysts, infarcts, and splenic hemorrhage may be seen with pancreatitis.




In about 7% to 12% of patients with acute pancreatitis, CT or MRI reveals free intraperitoneal fluid, usually in small volumes. Massive pancreatic ascites, caused by communication of a disrupted pancreatic duct with the peritoneal cavity, is rarely seen. This presentation is usually associated with a more severe form of pancreatitis. In most of these patients, characteristic pancreatic and peripancreatic abnormalities are recognized. Rarely, pancreatic ascites can develop in patients with a normal-appearing gland on CT scans. In these patients, paracentesis with amylase determination is needed to make the diagnosis. The treatment of pancreatic ascites includes conservative management with nasogastric suction, parenteral nutrition, and repeated paracenteses. If these measures fail, interventional or surgical procedures, including pancreatic duct stenting, pancreaticojejunostomy, and distal pancreatectomy, may be required.
Diagnostic Sensitivity.
The accuracy of CT in the diagnosis of acute pancreatitis depends to a large extent on the severity of the disease. The reported CT sensitivity for the diagnosis of acute pancreatitis ranges from 77% to 92%. The usefulness of CT is further supported by its high specificity. In most series, there are few false-positive findings, and CT specificity as high as 100% has been reported. In addition, by examining the entire abdomen, CT can reveal a variety of other abdominal conditions in patients clinically suspected of having acute pancreatitis.
Limitations in Diagnosis.
Limitations in the CT diagnosis of acute pancreatitis are related to suboptimal examinations resulting from poor technique, lack of intravenous contrast medium, or inability of the patient to cooperate. Motion or streak artifacts and paucity of retroperitoneal fat are limiting factors in some patients. In addition, in mild forms of clinical pancreatitis, morphologic parenchymal or retroperitoneal abnormalities do not develop and the gland may appear normal on CT scans. It occurs in patients with mild symptoms and transitory elevation of serum amylase concentration. The incidence of normal CT scans in these persons is not well established because surgical or pathologic correlation is lacking, but it has been estimated to be 14% to 28%. Experience has shown that given a good-quality CT scan, all patients with moderate or severe pancreatitis will exhibit some CT abnormality. In patients with normal results of CT examination, pancreatitis is either absent or of minimal clinical significance.
Staging.
By virtue of its ability to accurately and rapidly evaluate the pancreas, peritoneum, and retroperitoneum, CT can be used to predict the severity of acute pancreatitis. The revision of the Atlanta classification addresses the lack of uniformity between referring physicians and diagnostic radiologists in describing the terms related to acute pancreatitis. The classification addresses fluid collections before and after 4 weeks of the onset of symptoms by the presence or absence of necrosis and by the presence or absence of infection.
Grading System.
The CT severity index (CTSI), developed by Balthazar and colleagues, focused on the presence and degree of pancreatic inflammation and necrosis to predict morbidity and mortality in patients with acute pancreatitis. In 2004, Mortele and coworkers modified the index with improved correlation with patient outcomes. The modified scale ascribes points to degrees of pancreatic inflammation.
Grade A: normal pancreas
Grade B: focal or diffuse enlargement of the gland including contour irregularities and inhomogeneous attenuation but without peripancreatic inflammation
Grade C: intrinsic pancreatic abnormalities associated with inflammatory changes in the peripancreatic fat (see Figs. 97-4 and 97-5 )
Grade D: small and usually single, ill-defined fluid collection
Grade E: two or more large fluid collections or the presence of gas in the pancreas or retroperitoneum ( Fig. 97-14 )

Figure 97-14
Infected necrosis.
Axial contrast-enhanced MDCT image shows absence of normal enhancing pancreatic parenchyma. There are air bubbles and debris ( arrows ) in the pancreatic bed suggesting infected necrosis. The patient underwent pancreatic débridement.
In cases of pancreatic necrosis, points are given for certain degrees of necrosis, with more than 30% necrosis receiving the maximum 4 points. Two points are given for cases that involve extrapancreatic complications. The numerical modified CTSI has a maximum of 10 points and is the sum of inflammation points, pancreatic necrosis points, and extrapancreatic complication points.
Mortele and coworkers modified the CTSI as the score obtained did not significantly correlate with the development of complications and demonstrated significant interobserver variability in scoring. Moreover, Balthazar and colleagues found no significant difference in morbidity and mortality in differentiating between 30% to 50% necrosis and more than 50% necrosis. The modified CTSI condenses the scoring for necrosis to differentiate above and below 30% and also gives points for extrapancreatic complications.
Delineation of Necrosis.
During the arterial phase of bolus intravenous administration of contrast medium, the normal pancreas should enhance homogeneously. Mild inflammation and interstitial edema do not interfere with the expected homogeneous enhancement of the gland (see Figs. 97-1 and 97-3 ). When necrosis is present, there is absence of contrast medium enhancement together with liquefaction and a change in the density or signal intensity of the gland ( Figs. 97-15 and 97-16 ). Gadolinium-enhanced T1-weighted gradient-echo MR images demonstrate pancreatic necrosis as low signal areas of nonenhancing parenchyma ( Fig. 97-17 ). The process can be focal or segmental or can affect the entire pancreatic gland and may involve the peripancreatic tissue in combination with the pancreas or separately (see Figs. 97-15 to 97-17 ). In the original series of Kivisaari and associates using CT, mild pancreatitis was associated with a rapid rise in the density of the gland by 40 to 50 HU after administration of contrast material. Pancreatic necrosis was found at surgery in all patients who exhibited absence of enhancement or low enhancing values of less than 30 HU (see Figs. 97-15 and 97-16 ). Other investigators have corroborated the CT-surgical correlation in the detection of pancreatic necrosis. In a series of 93 patients, an overall CT accuracy of 85% with 100% sensitivity for extensive glandular necrosis was found. At times, MRI may be more sensitive than CT for the detection of pancreatic necrosis because of the greater sensitivity of MRI to gadolinium than of CT to iodinated contrast agents. Many patients with pancreatic necrosis, however, may be seriously ill and may be unable to hold the breath for the required MRI sequences.



In Balthazar’s series, there was good correlation between necrosis and length of hospitalization, development of complications, and death; patients without necrosis had no mortality and only 6% morbidity, whereas patients with necrosis exhibited 23% mortality and 82% morbidity. The degree of necrosis also was important. Patients with small areas of necrosis (<30%) showed no mortality and 40% morbidity, whereas large areas of necrosis (≥50%) were associated with 75% to 100% morbidity and 11% to 25% mortality. The combined morbidity of patients with more than 30% necrosis was 94%, and the mortality was 29%. There was no significant difference in prognosis between patients with up to 50% necrosis and those with more than 50% necrosis. Other studies have shown that infected pancreatic necrosis is also a significant predictor of organ failure and mortality in acute pancreatitis. Garg found a mortality rate of 12% for patients with sterile necrosis and 50% for those with infected necrosis.
Indications for Examination.
Many patients with acute interstitial edematous pancreatitis have typical clinical presentations and show rapid amelioration with limited supportive therapy. They do not require imaging examinations for diagnosis or management. On the other hand, CT imaging is needed and should be performed early when the clinical diagnosis is in doubt; there is failure to respond to medical treatment within 48 to 72 hours; acute abdominal symptoms (distention, tenderness), leukocytosis, or fever is present; patients have organ failure or signs of severe acute pancreatitis; and there is a change in clinical status suggesting a developing complication. Specific indications for performing MRI over CT include allergy to iodinated contrast agents; to detect the etiology of pancreatitis, such as choledocholithiasis, pancreas divisum, and pancreatic tumors; and to characterize complex fluid collections as liquefied or necrotic and to determine their drainability.
Complications
Local complications after acute pancreatitis include acute peripancreatic fluid collections, pseudocysts, acute necrotic collection, and walled-off necrosis. The prompt detection and treatment of local complications are essential because they are responsible for more than 50% of the mortality associated with acute pancreatitis.
Fluid Collections and Pseudocysts
Acute pancreatic fluid collections are generally seen within the first 4 weeks and pseudocysts after 4 weeks. Acute pancreatic fluid collections characteristically do not have a solid component, lack a discrete wall, and are located near the pancreas (see Fig. 97-13 ). Fluid collections develop in up to 50% of patients with acute pancreatitis within the first few days of pancreatitis. The fluid may be pancreatic juice, serum, or blood. They develop either from actual rupture of the pancreatic duct with liberation of enzymes and pancreatic juice or secondary to exudation of fluid from the surface of the pancreas due to activation of pancreatic enzymes within the gland. The fluid is contained by whatever structures happen to be adjacent to the collection. Most fluid collections are absorbed within 2 to 3 weeks. Drainage or aspiration is generally avoided to prevent introduction of infection unless it is thought to be a rare infected acute pancreatic fluid collection. Unresorbed fluid collections can organize and, within 4 to 6 weeks, develop a fibrous capsule, forming a pseudocyst. The lack of resorption of extravasated secretions and a communicating tract with the pancreatic ductal system are implicated in the development of pseudocysts.
When an acute pancreatic fluid collection becomes encapsulated by a fibrous wall more than 4 weeks after symptom onset, it is referred to as a pseudocyst. Pseudocysts often resolve spontaneously when they lose communication with the pancreatic duct. Pseudocysts develop during the initial attack of pancreatitis in 1% to 3% of patients. They have been reported to occur in 12% of patients after several episodes of alcoholic pancreatitis. The main causes of pancreatic pseudocysts are chronic alcoholism (75%) and abdominal trauma (13%), with cholelithiasis, pancreatic carcinoma, and idiopathic causes composing the remainder. If the patient with a suspected pseudocyst has no history of pancreatitis, pancreatic trauma, or pancreatic surgery, a follow-up imaging study or aspiration biopsy may be required to exclude a pancreatic cystic neoplasm.
The clinical significance of a pseudocyst is related to its size and the potentially lethal complications that may occur. Pseudocysts displace and compress adjacent abdominal organs and can produce obstruction, pain, and jaundice. Spontaneous rupture in the peritoneal cavity leads to pancreatic ascites or peritonitis. Erosion into an adjacent vessel leads to massive and sudden hemorrhage. Most of these complications, however, occur in pseudocysts larger than 4 to 5 cm. Small pseudocysts, often revealed by CT in asymptomatic patients, have a low incidence of morbidity and can be observed expectantly with clinical and CT examinations. Surgical, endoscopic, or percutaneous drainage is indicated for pseudocysts that increase in size, become symptomatic, or develop complications. The development of percutaneous and endoscopic techniques offers an alternative means of drainage of pseudocysts that is less invasive. Success rates of more than 90% have been reported with percutaneous drainage of pseudocysts guided by CT or sonography. Percutaneous drainage techniques of pancreatic fluid collections are discussed in Chapter 95 .
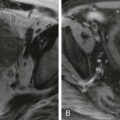
Pseudocysts vary greatly in size and are generally round or oval. On CT scans, they are characterized by low fluid density (<15 HU) contents and by a peripheral fibrous capsule. Higher attenuation values are indicative of secondary infection or the presence of necrotic tissue. Hounsfield unit values greater than 40 to 50 are suggestive of intracystic hemorrhage. On MRI, uncomplicated pseudocysts typically show low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. Complicated pseudocysts may present high signal intensity on T1-weighted images because of hemorrhagic or proteinaceous fluid and may contain solid debris; these are best depicted on T2-weighted images ( Figs. 97-18 and 97-19 ). MRI is superior to other imaging modalities to characterize the content of fluid collections, which may have prognostic and therapeutic significance.


ERCP is less effective than CT or MRI in showing pseudocysts as less than 50% of pseudocysts fill with contrast material at ERCP. Conversely, MRCP is able to detect pseudocysts that do not communicate with the pancreatic duct. ERCP, however, may be required to demonstrate communication between a pseudocyst and the pancreatic duct, although sometimes it can be seen on MRI ( Fig. 97-20 ). Communicating pseudocysts may require prolonged catheter drainage until the communication to the pancreatic duct closes and the cyst collapses. Decompression of concomitant pancreatic duct obstruction may be required.

Although many of the initial poorly defined fluid collections seen in acute pancreatitis resolve spontaneously, the natural history of a pseudocyst is difficult to predict. They may persist, they can resolve, or they can even continue to grow over time. Spontaneous resolution ( Fig. 97-21 ) even of large pseudocysts can occur and is explained by drainage into the pancreatic duct, erosion into an adjacent hollow organ (stomach, small bowel, colon), or rupture with spillage into the peritoneal cavity.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree