The imaging features of spinal parasitic diseases and other rare infections are herein discussed. These diseases are distributed worldwide, with increased prevalence in areas with poor sanitary conditions and in developing countries. In nonendemic areas, sporadic cases may occur, consequent to increased international travel and immunocompromising conditions. Infectious diseases are usually treatable, and early detection is often crucial. A thorough comprehension of the imaging patterns associated with the clinical features, epidemiology, and laboratory results allows the radiologist to narrow down the options for differential diagnosis and facilitates the timely implementation of appropriate therapies.
Key points
- •
Despite the reporting of uncommon diseases, often with similar patterns, infectious diseases are usually treatable, and radiologists may play a pivotal role in the appropriate diagnostic workup.
- •
The most common parasitic disease involving the central nervous system is cysticercosis; however, spinal cord schistosomiasis is also an important diagnosis in endemic regions. Additionally, toxoplasmosis is a common pathogen in immunocompromised patients.
- •
This article discusses key features of trypanosomiases and echinococcosis. Additionally, several nonparasitic diseases are herein discussed, including syphilis, Baggio-Yoshinari syndrome, paracoccidioidomycosis, and HTLV-1–associated myelopathy.
Introduction
A large number of agents can produce spinal lesions. Among these, bacteria are the most common agents, although other rarer organisms also can cause spinal injuries. Our current aim is to discuss the imaging features of spinal parasitic diseases and other rare infectious diseases, with an emphasis on spinal cord involvement. Despite the reporting of uncommon diseases, often with similar patterns, infectious diseases are usually treatable, and radiologists may play a pivotal role in the appropriate diagnostic workup by interfacing with clinicians and facilitating direct, appropriate testing for early diagnosis and correct treatment.
Despite the development of modern magnetic resonance (MR) techniques, these approaches are less useful for the detection of spinal cord involvement. Detailed analysis of structural images remains the main source of data for presumed diagnosis; however, diffusion-weighted images and constructive interference with the steady-state technique contribute to diagnosis in some specific cases herein described.
The most common parasitic conditions affecting the anatomic compartments of the spine are herein described. Subsequently, several rare infectious pathogenic agents are discussed individually with respect to their effect on the spine and its contents.
Introduction
A large number of agents can produce spinal lesions. Among these, bacteria are the most common agents, although other rarer organisms also can cause spinal injuries. Our current aim is to discuss the imaging features of spinal parasitic diseases and other rare infectious diseases, with an emphasis on spinal cord involvement. Despite the reporting of uncommon diseases, often with similar patterns, infectious diseases are usually treatable, and radiologists may play a pivotal role in the appropriate diagnostic workup by interfacing with clinicians and facilitating direct, appropriate testing for early diagnosis and correct treatment.
Despite the development of modern magnetic resonance (MR) techniques, these approaches are less useful for the detection of spinal cord involvement. Detailed analysis of structural images remains the main source of data for presumed diagnosis; however, diffusion-weighted images and constructive interference with the steady-state technique contribute to diagnosis in some specific cases herein described.
The most common parasitic conditions affecting the anatomic compartments of the spine are herein described. Subsequently, several rare infectious pathogenic agents are discussed individually with respect to their effect on the spine and its contents.
Parasitic diseases
Parasitic diseases are distributed worldwide, with an increased prevalence in areas with poor sanitary conditions and developing countries. Nevertheless, in nonendemic areas, sporadic cases may occur as a consequence of increased international travel and immunocompromising conditions. A variety of parasitic diseases can involve the central nervous system (CNS), with multiple clinical presentations. The most common of these diseases is cysticercosis, although in endemic regions, schistosomiasis is also a common cause of spinal cord syndrome. Additionally, toxoplasmosis is a frequent CNS pathogen in immunocompromised patients, particularly in patients with acquired immunodeficiency syndrome (AIDS), but it rarely involves the spinal cord.
Trypanosomiases and echinococcosis are also emerging parasitic diseases in some parts of the world. Although the definite diagnosis of a spinal parasitosis is usually confirmed based on histopathology, the clinical suspicion is generally based on a combination of ethnic, clinical, serologic, and neuroimaging features.
Neurocysticercosis
Overview
Cysticercosis is caused by implantation of the cestode Taenia solium (pork tapeworm) in humans, which serve as an intermediate host. This disease affects approximately 50 million people worldwide, with a prevalence of 3% to 6% of the population in endemic areas, including Central and South America, Eastern Europe, Africa, and some regions in Asia. Neurocysticercosis (NCC) has become an increasingly important emerging infection in the United States, largely due to the influx of immigrants from endemic regions.
Pathophysiology
Cysticercosis occurs as a result of ingestion of eggs by humans, which serve as incidental intermediate dead-end hosts. Embryos are released in the small intestine, which are subsequently lysed by gastric juices and invade the bowel wall to reach the arterial system. Then, the parasites lodge preferentially in neural and subcutaneous tissues, as well as in the skeletal muscles and ocular globes, where they continue to develop. In the CNS, the oncospheres develop into a secondary larval form called cysticerci over a period of 3 weeks to 2 months. The larval stage of this organism may become disseminated throughout all CNS compartments.
The most common larval form is Cysticercus cellulosae , which characteristically has a scolex and causes brain intraparenchymal lesions or, rarely, spinal intramedullary lesions. Cysticercus racemosae , which lacks a scolex, usually grows in grapelike clusters of thin-walled cysts and shows a tendency to predominate in the subarachnoid space. However, this terminology does not help with diagnosis from an imaging point of view because these organisms are both forms of the same parasite, commonly coexisting in a single patient.
The macroscopic appearance of cysticerci varies according to their location in the CNS. Cysticerci, in most cases, remain small, approximately 1 cm in diameter, and tend to lodge in the cerebral cortex or the basal ganglia. The Sylvian fissure and basal cisterns are the most common locations of subarachnoid cysticerci, where they may reach 10 cm or more ( Fig. 1 A).
Clinical manifestations
The clinical presentation of cysticercosis is often nonspecific, depending on the number and location of parasitic organisms and the ensuing inflammatory reaction. Degeneration of the cyst is usually associated with a mass effect and edema, resulting in focal neurologic symptoms; when this process occurs in the brain, seizures typically ensue.
Diagnosis
The proposed diagnostic criteria include epidemiologic factors and the results of physical examination, funduscopy, serologic tests, and imaging. Additionally, imaging plays a main role in confirming and fully characterizing the different forms of NCC.
Imaging
Four stages of development and regression have been described for NCC using both computed tomography (CT) and MR imaging, with histopathologic correlation ( Table 1 ). Notably, multiple anatomic sites are simultaneously involved, which is not uncommon in various stages of the disease. Severe forms of presentation with seizures or focal neurologic deficits are usually characterized on images by extensive vasogenic edema and contrast enhancement (colloidal or nodular-granular stages) and are represented by an isolated lesion in subcortical areas. Conversely, multifocal parenchymal lesions often do not elicit an inflammatory reaction, and most of the parasites in such cases remain alive. Subarachnoid cysticercosis lesions are often multiple and contain live parasites.
| First stage | |
| Vesicular | Cyst and scolex. |
| Second stage | |
| Colloidal | Ring enhancement and edema. |
| Third stage | |
| Nodular-granular | Decreased enhancement and edema. Initiation of calcification. |
| Fourth stage | |
| Calcified | Calcification on CT or MR imaging. a |
a Reactive inflammation in calcified lesions has been observed with recurrent edema and peripheral enhancement, mainly in a quiescent brain lesion.
Spinal neurocysticercosis
Extradural lesions are extremely rare, whereas spinal cord involvement is considered very uncommon and is reported in only 1.2% to 5.8% of patients with NCC. Furthermore, the leptomeningeal form occurs 6 to 8 times more often than the intramedullary form.
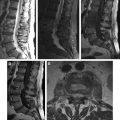
Presumably, intradural-extramedullary ( Fig. 2 ) involvement is a consequence of the downward migration of larvae from the intracranial compartment to the spinal subarachnoid space (see Fig. 1 B, C), and the cysts may remain mobile in this region ( Fig. 3 ). Similar to brain parenchymal lesions, intramedullary cysticercosis ( Fig. 4 ) typically arises from the hematogenous dissemination of larva, particularly in the thoracic cord, as a consequence of its vascular supply.
Neurologic manifestations may arise from an inflammatory reaction caused by parasitic metabolites, degenerated larva, the mass effect from intramedullary or extramedullary cysts (see Figs. 2 and 4 ), leptomeningitis (see Fig. 1 C; Fig. 5 ), or vascular insufficiency.
MR imaging is the most useful modality to evaluate spinal NCC, as this approach can reveal the intensity of the viable cystic fluid, which (whether in the spinal cord or the subarachnoid space) is usually similar to that of the cerebrospinal fluid (CSF) on both T1-weighted images (WIs) and T2WIs. MR further demonstrates mass effects, variable vesicular enhancement, and adjacent edema as a result of dead larvae. The absence of a CSF flow void is commonly observed adjacent to extramedullary cysts. High-resolution highly T2-weighted sequences, such as the use of constructive interference in steady-state (3-dimensional constructive interference in steady-state [3D-CISS]) techniques, allow for better delineation of the cyst and its scolex, when it is present (see Figs. 2 and 4 C). Cisternal MR imaging, as in myelography (see Fig. 3 ), enables detection of the cyst, which appears as a hypointense cystic wall surrounded by contrast material.
However, the MR imaging features of intramedullary NCC are not specific in the absence of the scolex, and the differential diagnosis includes neoplastic, inflammatory, demyelinating, vascular, and granulomatous lesions. Marked eosinophilia may be useful for differentiation from a spinal arachnoid cyst. Intramedullary NCC also may occur in conjunction with cysticercal meningitis and intracranial lesions, which should be recognized.
Schistosomiasis
Overview
Spinal schistosomiasis, the best-known form of neuroschistosomiasis, is a severe, underrecognized form of schistosomiasis that occurs at any time during the parasitic infestation.
Schistosomiasis is one of the most widespread parasitic diseases worldwide and is an important public health problem, particularly in tropical areas. Approximately 200 million people worldwide are afflicted with schistosomiasis, and approximately 20 million develop severe disease, including CNS forms. Neuroschistosomiasis has been increasingly reported not only in endemic areas but also in Western countries, owing to immigration and international travel.
Pathophysiology
Almost all reported cases of neuroschistosomiasis are caused by infection with Schistosoma mansoni , consequent to a parasite configuration that makes retrograde migration from the abdominal veins to Batson plexus difficult. Whereas Schistosoma haematobium primarily affects the urinary tract, Schistosoma japonicum has an increased likelihood of extending to the brain parenchyma.
Since the first description of this disease in the 1930s, approximately 800 cases have been reported, most of them due to S mansoni . Neurologic symptoms may result from the deposition of eggs surrounded by granulomatous reactions in circumscribed areas of the brain or spinal cord, although the simultaneous occurrence of cerebral and spinal schistosomiasis is extremely rare.
Clinical manifestations
In endemic areas, spinal schistosomiasis is more common in children, adolescents, and young adults; however, with the exception of hepatomegaly (present in 25% of the patients), these patients rarely show any other symptoms. The disease usually presents acutely or subacutely as conus medullary syndrome and is often associated with the involvement of cauda equina roots.
Clinically, spinal cord schistosomiasis may be classified into 3 classic forms (summarized in Table 2 ); however, the disease can progress from one form to another. The medullary form is generally associated with a rapid course and severe weakness. By contrast, in conus-cauda equina syndrome, the symptoms develop more slowly, the distribution of the sensorimotor alterations is predominantly asymmetric, and the muscle weakness is less severe.
| Clinical Forms | Clinical Manifestations | Imaging |
|---|---|---|
| Medullary: predominantly spinal cord involvement. | Rapid course, severe weakness, and a symmetric distribution of the sensorimotor abnormalities. May present with high eosinophil levels in the CSF. | T2 hyperintensity and enlargement of the spinal cord associated with mild and heterogeneous enhancement. |
| Conus-cauda equine syndrome: mainly conus medullaris or cauda equina involvement ( Fig. 6 ). | Symptoms develop more slowly, muscle weakness is less severe, and the distribution of the sensorimotor alterations is predominantly asymmetric. Usually presents with high eosinophil levels in the CSF. | Enlargement and heterogeneous gadolinium enhancement of the conus medullaris. Thickening of the cauda equina. |
| Myeloradicular: predominantly spinal cord and nerve root involvement ( Fig. 7 ). | This is the most common presentation and represents an intermediate form of presentation. Often begins with lumbalgia and pain in the lower limbs (radiculopathy) followed by muscular weakness and sensory disturbances in the lower limbs. Usually presents with high eosinophil levels in the CSF. | Thickening of the spinal nerve roots and leptomeningeal enhancement. Epidural venous plexus congestion might also be identified. |
Diagnosis
Laminectomy with biopsy of the nervous tissue is the only method that provides a definite diagnosis of spinal schistosomiasis; however, to prevent sequelae, this procedure should be avoided. Blood eosinophilia, antibody levels, and the presence of parasite ova in the urine and/or stool may not be detectable at disease onset. CSF examination usually reveals nonspecific abnormalities that may be found in other parasite infections, including eosinophils. The detection of antibodies to schistosomes in the CSF by enzyme-linked immunosorbent assay (ELISA) is specific for the diagnosis of S mansoni , although further validation of this technique has been recommended.
Although its clinical picture is nonspecific, spinal schistosomiasis should be strongly considered in young patients presenting with acute paraplegia, myeloradicular pain syndrome, or cauda equina syndrome with a positive epidemiologic origin. The clinical diagnosis becomes less likely when higher segments are affected or when the symptoms progress more slowly. A rapid and pronounced improvement after treatment lends further support to diagnosis. The differential diagnosis of spinal schistosomiasis should include ependymoma, spinal cord astrocytoma, metastatic tumors, and venous congestion in a spinal dural arteriovenous fistula.
Imaging
Although the alterations observed in cases of spinal schistosomiasis are usually nonspecific, MR imaging greatly contributes to the diagnosis, easily demonstrating the abnormalities in the spinal cord and helping to rule out the differential diagnosis. Although clinical forms usually coexist (see Table 2 ), the most common imaging findings are described in the medullary form and the conus syndrome (see Fig. 6 ), which is characterized by a patchy pattern hyperintensity on T2WIs, enlargement of the spinal cord, and a remarkable heterogeneous contrast enhancement on T1WIs, particularly in the lower cord and conus medullaris. This interesting multinodular enhancing pattern is associated with multiple schistosome eggs and granulomas in the spinal cord, peripheral contrast enhancement with eggs, and granulomas in the leptomeninges. In addition, this appearance may mimic a cord neoplasm.
Occasionally, MR imaging also may display an intramedullary arborized appearance, which is highly suggestive of this disease in individuals from endemic schistosomiasis regions. In cases with a longer evolution, medullary atrophy can be observed (see Fig. 6 D). In the other clinical forms (myeloradicular and cauda equine), MR imaging demonstrates thickening of the spinal roots (especially the cauda equina roots) and a linear radicular contrast enhancement, representing eggs and granulomas on the surface of nerve roots (see Fig. 7 ).
Rare forms
The granulomatous form of schistosomiasis results from an intense granulomatous inflammatory reaction around the eggs in association with gliosis and fibrosis. This reaction leads to the formation of focal expanding intra-axial or extra-axial lesions, which demonstrate a pattern of intense epidural enhancement adjacent to areas of medullary involvement. Extradural compromising (bilharzioma) is considered rare.
Other even rarer forms, which may be revealed by imaging, include acute transverse myelitis (which may be hemorrhagic and necrotizing) and an acute, anterior spinal artery syndrome.
Echinococcosis (Hydatid Disease)
Overview
Hydatid disease (echinococcosis) is a parasitosis caused by the larval stage of Echinococcus . Several carnivores and canines are definitive hosts for these parasites and can be found near homes in forest areas or the countryside. Humans are secondarily infected via the ingestion of food or water contaminated by eggs of the parasite. The 2 most frequent clinical forms are cystic echinococcosis, caused by Echinococcus granulosus , and, less frequently, alveolar echinococcosis, caused by Echinococcus multilocularis .
Hydatid disease remains a health problem in endemic areas of countries in which veterinary control is precarious, mostly in the temperate zones.
This disease is diagnosed by serologic and imaging tests. The most frequently involved organ is the liver, and CNS involvement is rare (1% to 2% of all cases), even in endemic areas.
Spinal echinococcosis: overview
Spinal involvement in echinococcosis is rare, although the thoracic segment of the spine is the most frequently affected region (50% of cases), followed by the lumbar (20%), sacral (20%), and cervical (10%) segments.
Spinal echinococcosis: clinical and imaging manifestations
The imaging and clinical presentation of spinal echinococcosis depends on the primarily infected anatomic structures. This disease usually manifests as isointense cystic lesions without significant peripheral edema. The fibrous capsule is characteristically hypointense on T2WIs and may display discrete peripheral enhancement in the presence of active inflammation ( Fig. 8 ).