Spinal involvement in human brucellosis is a common condition and a significant cause of morbidity and mortality, particularly in endemic areas, because it is often associated with therapeutic failure. Most chronic brucellosis cases are the result of inadequate treatment of the initial episode. Recognition of spinal brucellosis is challenging. Early diagnosis is important to ensure proper treatment and decrease morbidity and mortality. Radiologic evaluation has gained importance in diagnosis and treatment planning, including interventional procedures and monitoring of all spinal infections.
Key points
- •
Spinal involvement is common in human brucellosis.
- •
Osteoarticular disease and neurobrucellosis are the most common complications.
- •
Spinal brucellosis involves lumbar region in more than half of the cases.
- •
Preservation of vertebral architecture is typical.
- •
MR imaging is the currently the best imaging tool for diagnosis and follow-up in patients with spinal infections.
Introduction
Spinal brucellosis is a significant cause of morbidity and mortality, particularly in endemic areas. The diagnosis of spinal brucellosis is challenging but important to ensure proper treatment. Early diagnosis and treatment are crucial. Radiologic evaluations have gained importance in the diagnosis, evaluation, and treatment monitoring of all the spinal infections. Diagnosis can be made with imaging and isolation of the causative agent from blood, cerebrospinal fluid (CSF), or the lesion.
Introduction
Spinal brucellosis is a significant cause of morbidity and mortality, particularly in endemic areas. The diagnosis of spinal brucellosis is challenging but important to ensure proper treatment. Early diagnosis and treatment are crucial. Radiologic evaluations have gained importance in the diagnosis, evaluation, and treatment monitoring of all the spinal infections. Diagnosis can be made with imaging and isolation of the causative agent from blood, cerebrospinal fluid (CSF), or the lesion.
Definition and epidemiology
Brucellosis (undulant fever, Malta fever) is a zoonotic disease that effects animals as the primary host (ie, camels, sheep, goats) and humans as the secondary host. The infecting agent was first identified by Bruce in 1887 in a patient who died on the island of Malta. The disease is caused by small, nonmotile gram-negative facultative intracellular coccobacilli of the genus Brucella , including Brucella melitensis , Brucella abortus , Brucella suis , Brucella canis , and Brucella ovis , which are usually transmitted through the consumption of uncooked meat or unpasteurized dairy products.
B. melitensis is the most common microorganism isolated in brucella spondylitis and neurobrucellosis, which is also endemic in certain parts of the world. The incidence of spinal involvement in brucellosis is 2% to 65%. Men are affected more frequently than women, which may be reflective of occupational risks such as those encountered in the stock industry, which mainly employs men.
The type of skeletal involvement depends partly on the patient’s age and the Brucella species involved. Although arthritis, bursitis, tenosynovitis, and sacroiliitis are more frequently observed in younger patients, the frequency of spondylodiscitis increases with age, and its diagnosis may be difficult because brucella spondylitis may resemble many diseases that affect the spine, such as tuberculosis, pyogenic osteomyelitis, intervertebral disc herniation, and malignancy. Brucella spondylitis presents in focal and diffuse forms, commonly in people aged 50 and 60 years of age in endemic areas.
Spinal brucellosis suggests a spectrum of disease comprising infections of the numerous components of the spinal colon, including vertebral bodies (spondylitis), intervertebral discs (spondylodiscitis), facet joints (arthritis), ligaments, paraspinal soft tissues, epidural space (epidural phlegmon/abscesses), meninges, and subarachnoid space and the spinal cord itself (myelitis).
The lumbar region was noted to be the site of involvement in more than half of the cases, which was considered to be the result of its rich blood supply and higher likelihood of endplate degeneration. Thoracic vertebrae are involved in 19% of the cases. Cervical vertebrae are rarely affected, but this involvement is more dangerous because of potentially life-threatening complications, such as paraplegia and tetraplegia, in 1% of cases. Multilevel involvement of brucella spondylitis has been reported to occur in 6% to 36% of cases.
The World Health Organization estimates the worldwide incidence of new brucellosis cases to be more than 500,000 per year. Brucellosis is a common cause of vertebral osteomyelitis in geographic areas in which B. melitensis is endemic (ie, the Mediterranean basin, the Middle East, Latin America).
Mechanism
Brucellosis spreads hematogenously to tissues, and almost every organ can be affected. Microorganisms at a distant septic focus reach the spine by anterograde flow through the nutrient arterioles of the vertebral bodies or by retrograde flow through the paravertebral Batson venous plexus.
Brucella spondylitis usually begins in the superior endplate because of its rich blood supply, and causes bone destruction even in the early stages. Occasionally the inferior endplate may also be involved. The infection spreads to the remainder of the vertebral body along the medullary spaces. With the inflammatory process, the process of bone healing begins almost simultaneously, and frequently spills over in the form of anterior osteophyte, like “parrot’s beak.” Initially discs are spared, as they are in tuberculosis spondylitis. In the later stage, brucella spondylitis extends to the adjacent vertebrae through the intervertebral disc space. The intervertebral disc is involved as a secondary process.
Microorganisms may leak into the CSF with the help of an inflammatory vasculitic process, resulting in spondylitis and meningitis occurring simultaneously during the acute stage masking one’s symptoms over other’s. Epidural masses may accompany the whole pathologic process, sometimes causing compression of a nerve root or spinal cord, mimicking a herniated intervertebral disc. Facet involvement (6%–35%), intramedullary infections (1.2%–35.0%), and psoas abscess (1.2%–50.0%) are rare findings.
Intramedullary brucella infection or abscess is rare. The organism may act directly or indirectly through its endotoxins. The spinal cord or nerve root may be secondarily involved because of spondylitis, vasculitis, and arachnoiditis.
Immune-mediated demyelination has been proposed to explain certain chronic forms of neurobrucellosis. Impaired immune status is believed to be a risk factor for developing neurobrucellosis. Nervous system involvement in brucellosis might be from the persisting intracellular microorganisms or, perhaps, the infection triggering an immune mechanism, leading to neuropathology. In an experimental animal model, the ganglioside-like molecules expressed on the surface of B. melitensis were found to induce antiganglioside membrane 1 ganglioside antibodies, resulting in flaccid limb weakness and ataxia-like symptoms.
Infection triggers the immune-allergic mechanism, leading to myelopathy and/or a demyelinating state. The occurrence of inflammatory central and peripheral demyelination as the pathologic manifestation of some types of neurobrucellosis, associated with inflammatory perivenular infiltration but not with histologically demonstrable organisms, strongly suggests that autoimmune mechanisms play a role in some types of neurobrucellosis. This change in the spinal cord is virtually identical to that found in transverse myelitis or acute disseminated encephalomyelitis (ADEM), whereas the change that may be found in spinal roots may be identical to that found in acute inflammatory polyneuritis associated with Guillain-Barré syndrome. In these types of chronic neurobrucellosis cases, axons tend to be spared, although in severe cases, as in severe ADEM or Guillain-Barré syndrome, considerable axonal loss and associated peripheral neuroaxonal dissolution are seen. It is also possible that the occurrence of a hyperergic immune response after latency from a bout of acute brucellosis is not caused by the slowed autoimmune response during a phase of chronic infection, but rather reinfection. Chronic inflammatory changes may be found in the perineurium in patients with neurobrucellosis, and adhesive arachnoiditis may develop in the subarachnoid space. Perineural inflammation may lead to dysfunction of peripheral nerves, with resulting radiculitis syndromes, especially if these inflammatory changes progress to the point of granuloma formation. Recurrence of myelitis has also been reported.
Chronic Brucella infection develops because of either the difficulties inherent in killing an intracellular parasite or inadequate treatment, rendering a potentially deleterious autoimmune response to infection, or the hosts’ particular vulnerability to the immune response engendered by Brucella infection that have chronically infected other parts of the body or by reintroduction of infection to an already sensitized individual. Chronic brucellosis does not develop in all mistreated or untreated individuals; other host factors likely play a role in the susceptibility to chronic brucellosis, and these hosts usually harbor Brucella organisms in their lymphatic/reticuloendothelial system.
Brucella can cause vasculitis, either from bacterial proliferation in the vascular endothelium or from the actions of bacterial toxins. It has no predilection of size or location of vascular structure. Arterial and/or venous structures may be affected. These vascular changes may occur in large or smaller-caliber arteries and veins, resulting in focal or multifocal arterial occlusions; venous thrombosis; lacunar, hemorrhagic, or nonhemorrhagic infarctions; mycotic aneurysm formation; subarachnoid hemorrhages from ruptured mycotic aneurysms; and subdural hemorrhages.
Clinical manifestations
Brucellosis may appear in 4 different forms, namely acute, subacute, chronic, and relapsing. The symptoms vary according to the site of involvement and the stage of infection. The disease is characterized by a febrile illness, with severe rheumatism that occurs after an incubation period of 1 to 3 weeks.
Clinically, the disease usually presents with a wide range of nonspecific clinical signs and symptoms, such as fever, malaise, profuse night sweating, weight loss, polyarthralgia, generalized myalgia, and headache. Localized back pain is the earliest and the most important symptom of brucella spondylitis, and 10% to 43% of these patients have some degree of neurologic deficit.
Brucella is an intracellular bacterium that may remain dormant and reactivate after a variable period of clinical latency, possibly causing relapses. The triggering effect of reactivation is still unknown.
Morbidity rates increase with delayed diagnosis. Neurologic complications may appear from spinal involvement of the osteoarticular disease, such as epidural abscess, radiculoneuritis, myelitis, or demyelinating neuropathy, which are the main causes of morbidity in brucellosis.
Diagnosis
Brucellosis has a clinical course with nonspecific symptoms and signs. Laboratory results alone are not efficient in establishing a correct diagnosis. Mildly elevated sedimentation rates and liver function enzyme levels, lowered hemoglobin levels, thrombocyte counts, and white blood cell counts are seen in most cases.
A standard tube agglutination test using smooth suspension of killed bacterial antigen may be helpful for diagnosis. Agglutination titers of 1:80 for patients without history of animal contact and 1:160 for those with a history of animal contact are considered positive findings for infection. An enzyme-linked immunosorbent assay for IgG and IgM antibodies for Brucella may be used, with antibody levels more than 40 arbitrary unit (AU) regarded as positive findings of infection.
Definite diagnosis can be made primarily through isolation of the microorganism from blood, CSF, bone marrow or from the abscess itself. Image-guided procedures are helpful through this process, such as computed tomography (CT)–guided percutaneous biopsy. Blood culture results are not always positive for the bacteria (range, 35%–92%), because of the possible previous use of antimicrobial agents and the intermittent feature of the bacteremia. Serology may help establish a diagnosis based on positive (>1:160) titers of antibodies to Brucella or titers greater than 1:320 on Coombs test.
Hence, neurobrucellosis is the second most common complication of brucellosis and requires longer treatment; acute meningitis, myelitis, and myelopathy should be considered and screened for properly based on serum and CSF serology, quantitative changes in cerebrospinal CSF, and imaging studies. All of this information indicates that a precise imaging study is necessary for accurate and timely diagnosis. Spinal MR imaging examination is the gold standard among all radiologic methods of diagnosis.
Plain Radiographs
Plain radiography is an easily accessible and quick method of spinal imaging. It provides information mostly about disorders of the vertebral column. It is widely used in patients with back pain, but its sensitivity is very low, especially in the early stages of brucella spondylitis. Because brucella spondylitis is a slowly progressing disorder, only one-fourth of the patients have radiologic abnormalities on admission. Osteophytes, sclerosis, and osteoporosis of the vertebral body, narrowing of the intervertebral disc space are the common, nonspecific findings, usually seen in the lumbar spine, which resemble those seen in most degenerative disorders. Posterior elements are mostly preserved. No central necrosis is present and the vertebral body is mostly morphologically intact, which allows brucella spondylitis to be differentiated from tuberculous spondylitis.
Computed Tomography, Scintigraphy, and PET
CT is superior to plain radiographs in many ways. It provides detailed information about bony structures through the capability of 3-dimensional imaging. With the help of intravenous contrast agents, CT can also provide information about paraspinal soft tissue and spinal canal masses. Although it is not sensitive, and differential diagnosis from noninfectious or tumoral lesions may be difficult, it may be useful in image-guided percutaneous procedures, such as biopsy of the infected area, to confirm the diagnosis or aspiration of the paravertebral abscess.
Intrathecal injection of contrast agents during CT scan or conventional myelography is contraindicated in spinal infections, because it may cause epidural infection to extend into the subarachnoid space, possibly resulting in meningitis.
Bone scan with technetium 99m is rather a specific method for detecting osteomyelitis, discitis, and aseptic spinal diseases. Diffuse-form brucella spondylitis shows prominently increased extended uptake, whereas focal-form shows moderately increased uptake only in the anterior part of the vertebral body. Skeletal scintigraphy offers coarse anatomic detail and limited tissue resolution, which in turn necessitates the use of an advanced imaging method, such as MR imaging.
PET scan is another alternative method that may become useful in differentiating infectious and degenerative disorders of vertebral endplates. High 18-F Fluorodeoxyglucose (FDG)-Positron emission tomography uptake is seen in spondylitis, whereas no or low FDG uptake is evident in degenerative disorders.
MR Imaging
MR imaging is currently the best imaging tool for diagnosis and follow-up in patients with spinal infections. It has higher sensitivity and specificity and provides more comprehensive anatomic detail, especially in the soft tissue compartments (ie, intervertebral discs, epidural space, paraspinal soft tissues, spinal canal, spinal cord, nerve roots) compared with other imaging methods.
Two radiologic forms of brucella spondylitis exist: the focal form, which is limited to the anterior part of the endplate, and the diffuse form, which involves the entire vertebral body, intervertebral disc, adjacent vertebra, epidural space, meninges, and spinal cord.
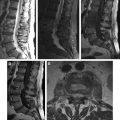
Osteoarticular disease is the most common complication of brucellosis. The earliest reaction in the vertebral body is the accumulation of water in the marrow, which can be detected easily by MR imaging. Even though bone marrow edema is characterized by low signal intensity on T1-weighted images (T1WI) and mainly high signal intensity on T2-weighted images (T2WI) or fluid-attenuated inversion recovery (FLAIR) images, fat-saturated short tau inversion recovery (STIR), fat-saturated T2WI, or spectroscopic inversion recovery sequences are more reliable and can detect the very early changes in the infected vertebral bodies ( Fig. 1 ).
The early form of brucella spondylitis is characterized by the lysis of the anterior aspect of the superior endplate at the discovertebral junction ( Fig. 2 ). Bone healing begins almost simultaneously with the inflammatory process and frequently results in the formation of an anterior osteophyte (parrot’s beak), and sclerosis, but the vertebral body may remain intact through the process. Generally, the spine stabilizes itself with sclerosis and bony ankylosis in chronic brucella spondylitis, whereas vertebral collapse, angulation, and scoliosis are rare (see Fig. 2 ). Abnormal segmentation must be differentiated from bony ankylosis, because it can be mistaken due to intact architecture and anterior scalloping of vertebra bodies in brucellosis. T1WI is generally helpful to show these morphologic abnormalities. Infected vertebral bodies, endplates, and involved facet joints show enhancement on fat-suppressed postcontrast T1WI. However, it is difficult to differentiate lesions occurring in patients with known tumor, trauma, degenerative disease, prior spinal surgery, initial stages of infection, preexisting narrowed disc space, hematologic disorders, and chronic diseases. Especially in the lumbar cases, the peritoneum adjacent to the involved vertebrae may be thickened and periaortic lymph nodes enlarged.