Part 5: Arthropathies
Case 75

Clinical History
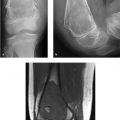
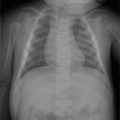
A 45-year-old man with bilateral foot swelling, redness, and warmth, but no history of trauma or pain (Fig. 75‑1).
Key Finding
Intense, diffuse, nontraumatic bone and soft-tissue edema centered at the midfoot.
Top 3 Differential Diagnoses
Charcot arthropathy: Charcot arthropathy, also referred to as neuropathic osteoarthropathy, is secondary to neuropathy. Reduced proprioception and pain sensation are associated with repetitive microtrauma and alterations in the sympathetic nervous system. Charcot foot is most commonly seen in patients with a history of diabetes mellitus (type I or II) for at least 10 years, but can be due to other causes (e.g., neurosyphilis). Charcot foot can be missed by over 90% of providers prior to foot specialist referral, and it is often debilitating when it is not managed effectively in its early stages.
Charcot arthropathy classically involves the midfoot, with involvement of the tarsometatarsal and tarsal joints in at least 80% of cases. The “active phase” is characterized initially by inflammation, followed by osseous fragmentation and collapse of the longitudinal arch of the foot. These imaging findings precede more extensive osteoarticular destruction (often with bone proliferation) and deformity (e.g., rocker-bottom foot).
Radiographs may be normal or nondiagnostic during the early stage of Charcot foot. MRI can be useful for early diagnosis, monitoring disease activity, and assessing complications, such as infection.
Infection: Cellulitis and osteomyelitis commonly cause intense edema in the soft tissues and bone marrow of the foot. Since bone edema on MRI is entirely nonspecific, soft-tissue defects should always be sought. In the absence of deep ulceration or penetrating trauma, foot osteomyelitis is rare. Notably, soft-tissue ulcers may be seen in some patients with Charcot foot, and therefore osteomyelitis and Charcot foot may coexist. In addition to deep ulceration and cellulitis, other MRI findings associated with osteomyelitis may include a sinus tract, abscess, and cortical erosion.
Clinically, high-laboratory inflammatory marker levels (e.g., erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]) are more consistent with acute infection rather than Charcot foot.
Arthritis: Arthritis, especially osteoarthritis and gout, may target the midfoot and cause bone edema. Clinically, osteoarthritis and gout are not associated with warmth and peripheral neuropathy (unlike Charcot foot).
With imaging of osteoarthritis, typical findings of degenerative joint disease are seen, including osteophytes and subchondral cysts. With gout, tophi and osseous erosions with overhanging edges are characteristic. With both osteoarthritis and gout, bone fragmentation with severe malalignment (which are typical of advanced Charcot foot) are generally absent.
Diagnosis
Charcot arthropathy (neuropathic osteoarthropathy).
Pearls
Bone marrow edema is a nonspecific finding that may be due to inflammation, infection, and/or altered biomechanics.
In the foot, unless there is a deep ulcer or penetrating trauma, osteomyelitis is almost never present.
Osteoarthritis and gout are known to affect the midfoot, but imaging findings are generally different from Charcot foot and osteomyelitis.
Suggested Readings
- 233 Dodd A, Daniels TR. Charcot neuroarthropathy of the foot and ankle.. J Bone Joint Surg Am 2018; 100 (8) 696-711 PubMed 29664858
- 234 Holmes C, Schmidt B, Munson M, Wrobel JS. Charcot stage 0: A review and considerations for making the correct diagnosis early.. Clin Diabetes Endocrinol 2015; 1 (1) 18 PubMed 28702236
- 235 Rosskopf AB, Loupatatzis C, Pfirrmann CWA, Böni T, Berli MC. The Charcot foot: a pictorial review.. Insights Imaging 2019; 10 (1) 77 PubMed 31385060
- 236 Schmidt BM, Holmes CM. Updates on diabetic foot and Charcot osteopathic arthropathy.. Curr Diab Rep 2018; 18 (10) 74 PubMed 30112582
Case 76

Key Finding
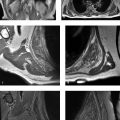
Polyarticular arthritis in an adolescent, with “blooming” intra-articular hemosiderin.
Top 3 Differential Diagnoses
Juvenile idiopathic arthritis (JIA): JIA refers to heterogeneous forms of arthritis of unknown etiology that begin prior to the age of 16 years and persist for more than 6 weeks. JIA is considered the most common rheumatic disease in children (with population prevalence similar to type 1 diabetes mellitus).
Although JIA nomenclature continues to evolve as more is understood about these idiopathic conditions, the major subtypes of JIA currently include systemic arthritis, polyarthritis, oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis.
JIA can affect essentially any joint, including the knee, ankle/foot, and wrist/hand. Imaging is important in early diagnosis (e.g., ruling out other disorders that may mimic JIA) and assessing risk of erosive damage. If initial treatments fail, patients often benefit from more aggressive pharmacologic treatment (e.g., disease-modifying antirheumatic drugs [DMARDS]) during a therapeutic “window of opportunity,” before advanced arthritis occurs. If untreated, growth disturbances may occur (e.g., hyperemia can be associated with epiphyseal overgrowth and premature fusion of physes).
Both MRI and sonography may be complementary to clinical examination and radiography by showing subclinical synovitis, erosions, and enthesitis. MRI is the only imaging method that detects bone marrow edema, which is an important independent predictor of erosive damage and functional impairment.
Hemophilic arthropathy: Hemophilia can cause spontaneous, recurrent intra-articular hemorrhages that cause arthropathy. This X-linked inherited disorder only occurs in males.
The most commonly targeted joints include the knees, ankles, and elbows. The later stages of hemophilic arthropathy are well-evaluated by radiography, including epiphyseal overgrowth/deformity and diffuse joint space loss. In the early stages, however, radiography underestimates the extent of joint involvement compared to MRI.
MRI shows findings that resemble an inflammatory arthritis (e.g., JIA), including effusion and synovitis, followed by erosion and cartilage destruction. With hemophilic arthropathy (like JIA), multiple joints are often involved. However, with hemophilic arthropathy (unlike JIA), MRI can show low-T2 signal in joints that “bloom” with gradient-echo MRI. The differential diagnosis of hemosiderin confined to a single joint includes tenosynovial giant cell tumor (formerly termed pigmented villonodular synovitis), which is a monoarticular process.
Infectious arthritis: The differential diagnosis of arthritis can be extensive. Any inflammatory arthritis can show imaging findings associated with periarticular soft-tissue swelling, effusion, periarticular demineralization, erosions, and uniform joint space narrowing.
Infectious arthritis is particularly important to consider when there is an unexplained inflammatory arthritis confined to a single joint. However, in as many as 20% of cases, multiple joints may be involved. Infectious arthritis typically is caused by hematogenous spread of bacteria (e.g., Staph aureus).
The joints most frequently affected by septic arthritis in children include the hip and knee; other joints like the ankle and elbow are less common. Imaging shows nonspecific findings, including joint effusion and synovitis, periarticular edema, periarticular osteopenia, erosion, and eventually joint space narrowing. Therefore, if a joint effusion is demonstrated in a patient clinically suspected of an infectious arthritis, then joint aspiration should be performed to assess for an infectious etiology.
Diagnosis
Hemophilic arthropathy.
Pearls
If arthritis involves multiple joints, a systemic arthritis must be considered.
If arthritis involves multiple joints and intra-articular hemosiderin is present, consider hemophilia causing hemophilic arthropathy.
If arthritis affects multiple joints for more than 6 weeks in a patient younger than 16 years, consider JIA (a diagnosis of exclusion).
Suggested Readings
- 237 Jacobson JA, Girish G, Jiang Y, Sabb BJ. Radiographic evaluation of arthritis: degenerative joint disease and variations.. Radiology 2008; 248 (3) 737-747 PubMed 18710973
- 238 Malattia C, Tzaribachev N, van den Berg JM, Magni-Manzoni S. Juvenile idiopathic arthritis-the role of imaging from a rheumatologist’s perspective.. Pediatr Radiol 2018; 48 (6) 785-791 PubMed 29766250
- 239 Sudoł-Szopińska I, Jans L, Jurik AG, Hemke R, Eshed I, Boutry N. Imaging features of the juvenile inflammatory arthropathies.. Semin Musculoskelet Radiol 2018; 22 (2) 147-165 PubMed 29672804
- 240 von Drygalski A, Moore RE, Nguyen S et al. Advanced hemophilic arthropathy: sensitivity of soft tissue discrimination with musculoskeletal ultrasound.. J Ultrasound Med 2018; 37 (8) 1945-1956 PubMed 29363781
- 241 Wyseure T, Mosnier LO, von Drygalski A. Advances and challenges in hemophilic arthropathy.. Semin Hematol 2016; 53 (1) 10-19 PubMed 26805902
Case 77

Clinical History
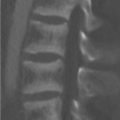
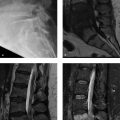
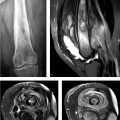
A 43-year-old woman was diagnosed with “chronic pain syndrome” for 15 years (Fig. 77‑1).
Key Finding
Bilateral sacroiliitis.
Top 3 Differential Diagnoses
Spondyloarthritis: Spondyloarthritis is a group of a chronic inflammatory rheumatic diseases that include ankylosing spondylitis (most common), psoriatic arthritis, reactive arthritis, and inflammatory bowel disease-associated arthritis. The differentiation into these types has become less important in light of the 2019 Assessment in SpondyloArthritis International Society classification criteria. For the past decade, spondyloarthritis has been generally classified into two forms, based on its main clinical manifestation– axial and peripheral.
Axial spondyloarthritis primarily targets the sacroiliac (SI) joints and spine, commonly presenting in patients aged < 40–45 years with inflammatory back pain (IBP). IBP is defined as presence of back pain with four out of five of the following parameters: age of onset < 40 years, insidious onset, improvement with exercise, no improvement with rest, and pain at night (improved by getting up).
Population prevalence in the USA is approximately 1%. Delay in diagnosis is a major problem (averaging up to 14 years), and therefore familiarity with diagnostic criteria is important. It is currently recommended that patients should be referred to a rheumatologist if ≥ one of three features of spondyloarthritis are present (IBP, HLA-B27 positivity, imaging evidence of sacroiliitis). Although serum biomarkers are commonly used (HLA-B27 and C-reactive protein), MRI of the SI joints is considered the most sensitive imaging biomarker. MRI facilitates earlier diagnosis and earlier treatment, which may include exercise, nonsteroidal anti-inflammatory drugs (NSAIDs), and biologic drugs (disease-modifying antirheumatic drugs [DMARDs]). Axial spondyloarthritis usually starts in the SI joints and only later involves the spine.
Ankylosing spondylitis is the most common cause of bilateral sacroiliitis. In the classic description of spondyloarthritis, sacroiliitis is characteristically symmetrical with ankylosing spondylitis and inflammatory bowel disease-associated arthritis, whereas with psoriatic and reactive arthritis, sacroiliitis may be unilateral, asymmetrical, or symmetrical.
Sacroiliitis-like changes: The SI joints can be affected bilaterally by a wide variety of mechanical and systemic disorders affecting other synovial or fibrous joints.
Mechanical (e.g., degenerative or posttraumatic) changes involving the SI joints are frequent. Most commonly, these include osteoarthritis, osteitis condensans ilii, and stress reaction. In addition to imaging features, clinical context and laboratory markers are helpful in differentiating true inflammatory sacroiliitis from mechanical changes.
Bilateral sacroiliitis caused by spondyloarthritis must be differentiated from systemic or metabolic conditions. Hyperparathyroidism causes subperiosteal resorption in a distribution that is bilateral and greater on the iliac side of the joint (like ankylosing spondylitis), but it does not cause joint space narrowing or ankylosis. Calcium pyrophosphate disease (CPPD) is seen in older patients with chondrocalcinosis who may develop bilateral SI joint subchondral cysts/erosions, sclerosis, and joint space narrowing.
Anatomical variations: Anatomical and physiological variations in the SI joints may be considered in the differential diagnosis of sacroiliitis and mechanical changes involving the SI joints.
Anatomical variations occur most commonly in women, and they can be seen in almost one-third of SI joint MRI examinations. These variations can be unilateral (28%) or bilateral (72%), and they can involve the cartilaginous or ligamentous part of the SI joint. Of five different types of anatomical variations, the “dysmorphic SI joint” (prevalence, 17%) and the “accessory SI joint” (prevalence, 11%) are most frequently associated with edematous and/or structural changes that could be mistaken for inflammatory sacroiliitis.
Diagnosis
Axial spondyloarthritis (ankylosing spondylitis).
Pearls
Bilateral sacroiliitis is associated with axial spondyloarthritis, most commonly ankylosing spondylitis.
The SI joints may be affected bilaterally by conditions that may mimic sacroiliitis, particularly mechanical (e.g., degenerative or posttraumatic) changes and systemic disorders (e.g., hyperparathyroidism or CPPD).
Anatomical variations can be seen in almost one-third of SI joint MRI examinations, and they may be associated with edematous and/or structural changes that could be confused with sacroiliitis.
Suggested Readings
- 242 Bray TJP, Jones A, Bennett AN et al. Recommendations for acquisition and interpretation of MRI of the spine and sacroiliac joints in the diagnosis of axial spondyloarthritis in the UK.. Rheumatology (Oxford) 2019; 58 (10) 1831-1838
- 243 Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities.. Clin Rheumatol 2019; 38 (3) 625-634 PubMed 30588555
- 244 El Rafei M, Badr S, Lefebvre G et al. Sacroiliac joints: anatomical variations on MR images.. Eur Radiol 2018; 28 (12) 5328-5337 PubMed 29876707
- 245 Eshed I, Hermann KA, Zejden A, Sudoł-Szopińska I. Imaging to differentiate the various forms of seronegative arthritis.. Semin Musculoskelet Radiol 2018; 22 (2) 189-196 PubMed 29672807
- 246 Maksymowych WP, Lambert RG, Østergaard M et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group.. Ann Rheum Dis 2019; 78 (11) 1550-1558 PubMed 31422357
- 247 Tsoi C, Griffith JF, Lee RKL, Wong PCH, Tam LS. Imaging of sacroiliitis: current status, limitations and pitfalls.. Quant Imaging Med Surg 2019; 9 (2) 318-335 PubMed 30976556
Case 78

Key Finding
Contour defect at the posterolateral humeral head.
Top 3 Differential Diagnoses
Erosive arthritis: In patients with inflammatory arthritis, the shoulder is often involved (up to approximately two-thirds of the patients with rheumatoid arthritis and one-third of patients with chronic ankylosing spondylitis). Shoulder involvement can include rotator cuff pathology, synovitis, and erosion.
With ankylosing spondylitis, the shoulder joint is the second most frequently targeted peripheral joint (after the hip joint). In some patients with ankylosing spondylitis, the entire superolateral/posterolateral aspect of the humeral head becomes eroded. This erosive process results in a characteristic defect of the humeral head which can mimic a Hill–Sachs lesion, which has been termed the “hatchet sign”.
Hill–Sachs lesion: A Hill–Sachs lesion is an impaction fracture deformity that occurs in at least two-thirds of anterior shoulder dislocations. The posttraumatic deformity is located at the posterosuperolateral aspect of the humeral head.
The Hill–Sachs lesion is often defined as significant (“engaging”) if it is in a position that allows it to engage with the anterior glenoid when the arm is abducted 90° and externally rotated 90°. Engagement of a Hill–Sachs lesion is a better predictor of recurrent instability than defect size alone. Although this topic continues to be an active area of investigation, engaging Hill–Sachs lesions are generally observed to be off the glenoid track (the glenoid track = 0.83 D – d, where D is the diameter of the inferior glenoid and d is the bone loss).
In addition to treating any glenoid injury (e.g., Bankart lesion), an engaging Hill–Sachs lesion may be treated with a remplissage procedure, which involves filling of the Hill–Sachs defect with the joint capsule and infraspinatus tendon (i.e., arthroscopic posterior capsulodesis and infraspinatus tenodesis, respectively).
Cystic lesion in the posterosuperior humeral head: Numerous types of cystic-appearing foci may occur in the bone at the margin of the humeral head. Humeral head cysts have been associated with many articular disorders and rotator cuff disorders. In addition, incidental cystic changes close to the bare area of the humerus, associated with degenerative changes and vascular channels at the posterolateral humeral head, also are commonly observed. Finally, in the posterosuperior portions of the humeral head (i.e., at the bare area or just posterior to the greater tuberosity), tiny (≤ 4 mm) pseudocysts lined with collagen connective tissue may occur and are thought likely to be a normal variant.
Diagnosis
Erosive arthritis (hatchet sign due to ankylosing spondylitis).
Pearls
With ankylosing spondylitis, a characteristic pattern of erosion at the superolateral/posterolateral aspect of the humeral head termed the “hatchet sign” can mimic a Hill–Sachs lesion.
A Hill–Sachs lesion is an impaction fracture deformity that occurs frequently after a shoulder dislocation. In some cases, it may be implicated in recurrent dislocations and treated surgically.
In the posterosuperior portions of the humeral head, tiny pseudocysts are likely an incidental normal variant.
Suggested Readings
- 248 Fox JA, Sanchez A, Zajac TJ, Provencher MT. Understanding the Hill–Sachs lesion in its role in patients with recurrent anterior shoulder instability.. Curr Rev Musculoskelet Med 2017; 10 (4) 469-479 PubMed 29101634
- 249 Gyftopoulos S, Beltran LS, Bookman J, Rokito A. MRI evaluation of bipolar bone loss using the on-track off-track method: a feasibility study.. AJR Am J Roentgenol 2015; 205 (4) 848-852 PubMed 26397335
- 250 Jin W, Ryu KN, Park YK, Lee WK, Ko SH, Yang DM. Cystic lesions in the posterosuperior portion of the humeral head on MR arthrography: correlations with gross and histologic findings in cadavers.. AJR Am J Roentgenol 2005; 184 (4) 1211-1215 PubMed 15788596
- 251 Maio M, Sarmento M, Moura N, Cartucho A. How to measure a Hill–Sachs lesion: a systematic review.. EFORT Open Rev 2019; 4 (4) 151-157 PubMed 31057952
- 252 Sankaye P, Ostlere S. Arthritis at the shoulder joint.. Semin Musculoskelet Radiol 2015; 19 (3) 307-318 PubMed 26021591
- 253 Soker G, Bozkirli ED, Soker E et al. Magnetic resonance imaging evaluation of shoulder joint in patients with early stage of ankylosing spondylitis: A case-control study.. Diagn Interv Imaging 2016; 97 (4) 419-424 PubMed 26612668
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree