2. Pathogenesis
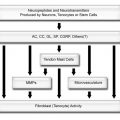
French anatomist and surgeon, Jean-François Jarjavay is credited as the first to describe morbid processes of the SSB in 1867.9 Since then, histologic studies have documented that synovial membrane may undergo inflammatory and/or degenerative changes and many now believe that they correspond to different stages in the spectrum of disease, with long-lasting inflammation leading to degeneration and fibrosis.10 When inflammatory infiltrate does occur in the bursal wall, it consists of mononuclear cells containing CD2-positive T lymphocytes but not granulocytes (see above).11,12 The increase in the number of intimal cells is mediated mainly by monocyte influx from the vascular compartment under cytokine and cell adhesion molecule control.13,14 Pathological increase in bursal fluid may result from (1) increased mechanical stress, with normal composition of synovial fluid and excessive production of hyaluronan by intimal type B synoviocytes or (2) inflammatory synovitis, with accumulation of exudate from increased vascular permeability in synovial membrane. In practice, bursal disorders are likely to result from a combination of the above and their relative contributions may not be as distinct as originally thought.15
An abnormal bursa can be observed in a variety of disorders, including local insults (e.g.: subacromial impingement, infection), involvement of adjacent structures (e.g.: full-thickness rotator cuff tear), and systemic diseases (e.g.: chronic inflammatory arthropathies). Whatever the cause, the pattern of shoulder pain is nonspecific because associated lesions elsewhere in the shoulder are frequent. The most common associated lesions are located at the rotator cuff tendons, and bursal disease may be the main source of pain in rotator cuff disorders.16,17 The hypothesis of an inflammatory reaction in the bursa causing or aggravating already installed damage to the collagen fibers of rotator cuff tendons is also attractive, but not yet established based on current data.
In recent years, several researchers investigated the role of the nociceptors located in the SSB in the generation of shoulder pain. The potential for chemical irritation is supported by studies that have demonstrated high concentrations of substances that may stimulate these nociceptors, including substance P and calcitonin gene-related peptide,18,19 matrix metalloproteinases,20 cyclooxygenase enzymes,20 tenascin-C,21 and the cytokines IL-1β, IL-6,10,20 as well as vascular endothelial growth factor, tumor necrosis factor, transforming growth factor, and basic fibroblast growth factor.10,22 In the synovial membrane, tenascin probably characterizes repair and thus a different phase of reaction than fibrosis.21 Although the mechanism is complex, there appears to be a positive correlation between the experience of shoulder pain and the amount of pro-inflammatory chemicals in the synovial membrane.23 Also, inflammatory cytokines produced by the synovial membrane may induce hyperalgesia, which is characterized by intensified pain with a reduced threshold to somatic stimulation even in the absence of inflammatory cell infiltrate.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree