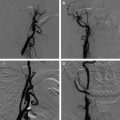
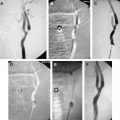
Atherosclerotic plaque at the carotid bifurcation is the primary cause of ischemic strokes and the degree of carotid stenosis is strongly associated with stroke risk in symptomatic patients. Stroke is the third-leading cause of death in the United States, constituting approximately 700,000 cases each year. In this article, the authors discuss the natural history of carotid and intracranial atherosclerosis, based on their broader knowledge of coronary atherosclerosis. Early to more advanced progressive lesions of the carotid are categorized, based on descriptive morphologic events originally cited for the coronary circulation. The histologic features associated with symptomatic and asymptomatic carotid disease are also addressed, along with the issues surrounding current stent-based therapies for the prevention of major recurrent vascular events.
Atherosclerotic plaque at the carotid bifurcation is the primary cause of ischemic strokes and the degree of carotid stenosis is strongly associated with stroke risk in symptomatic patients . Stroke is the third-leading cause of death in the United States, constituting approximately 700,000 cases each year, of which about 500,000 are initial attacks and 200,000 are recurrent. Ischemic stroke accounts for the largest number of new strokes (88%), followed by intracerebral hemorrhage (9%) and subarachnoid hemorrhage (3%) . Histopathologic studies of symptomatic and asymptomatic patients with carotid disease have identified specific lesion characteristics associated with cerebral ischemia, and the underlying mechanisms of plaque instability in the carotid share remarkable similarities with the coronary vasculature . In addition to the degree of carotid artery stenosis, the underlying plaque morphology is an important predictor of stroke risk. Intravascular ultrasound and MR imaging studies have shown that echolucent and lipid-rich plaques are associated with plaque rupture . In fact, plaque morphology is considered an additional independent predictor of cerebral infarction.
In this article, the authors discuss the natural history of carotid and intracranial atherosclerosis, based on their broader knowledge of coronary atherosclerosis. Early to more advanced progressive lesions of the carotid are categorized, based on descriptive morphologic events originally cited for the coronary circulation. The histologic features associated with symptomatic and asymptomatic carotid disease are also addressed, along with the issues surrounding current stent-based therapies for the prevention of major recurrent vascular events.
Lesion development at the carotid bifurcation
The earliest pathologic studies in man described the predilection of atherosclerosis near branch ostia, bifurcations, and bends, suggesting that important components of flow dynamics play an important role in its initiation and development . Atherosclerotic plaque tends to form at regions where flow velocity and shear stress are reduced, in particular at the carotid bifurcation, where disturbances in blood flow deviate from a laminar unidirectional pattern. Plaque burden is greatest on the outer wall of the proximal segment and sinus of the internal carotid artery, in the region of lowest wall shear stress. In marked contrast, the flow divider at the junction of the internal and external carotid arteries, a region exposed to the highest wall shear stress, is protected from atherosclerosis . Thus, the unique geometry and flow properties presented by the carotid bifurcation contribute to the degree and type of atherosclerotic plaque where the underlying morphology often outweighs the actual degree of stenosis, as the most critical determinant of clinical events.
Morphologic features of carotid atherosclerosis
Various plaque morphologies thought responsible for the onset of symptomatic carotid disease have been identified. Because the overall lesion types in the carotid vasculature share the common features of advanced atherosclerosis in coronaries, it seems appropriate that schemes developed to describe the evolution of coronary artery disease may be applied to the carotid, albeit with appropriate modifications. The American Heart Association conventionally uses a numeric classification to stratify various coronary lesion types . This scheme, however, implies an orderly, linear pattern of lesion progression, based on the assumption that all thrombosis occurs from plaque rupture, which later became refuted by the authors’ laboratory and others .
The authors’ laboratory subsequently developed a modified classification scheme guided by American Heart Association recommendations, based on the culmination of pathologic findings in more than 200 cases of sudden coronary death ( Box 1 ) . These defined morphologic criteria described for coronary arteries can be applied equally to carotid plaques and may serve as a unifying paradigm to understand the evolution of all atherosclerotic lesions, independent of the vascular bed.
Early nonsymptomatic carotid disease
Diffuse intimal thickening
Intimal xanthoma
Intermediate lesion
Pathologic intimal thickening
Progression of atherosclerosis leading to plaque enlargement
Plaque hemorrhage (± calcification)
Thin cap fibroatheroma (± calcification)
Lesions with thrombi
Plaque rupture with luminal thrombus
Plaque rupture with ulceration
Plaque rupture with organizing thrombus
Plaque erosion
Calcified nodule
Stable atherosclerotic plaque
Healed rupture/erosion
Fibrocalcific plaque
Total occlusion
Morphologic features of carotid atherosclerosis
Various plaque morphologies thought responsible for the onset of symptomatic carotid disease have been identified. Because the overall lesion types in the carotid vasculature share the common features of advanced atherosclerosis in coronaries, it seems appropriate that schemes developed to describe the evolution of coronary artery disease may be applied to the carotid, albeit with appropriate modifications. The American Heart Association conventionally uses a numeric classification to stratify various coronary lesion types . This scheme, however, implies an orderly, linear pattern of lesion progression, based on the assumption that all thrombosis occurs from plaque rupture, which later became refuted by the authors’ laboratory and others .
The authors’ laboratory subsequently developed a modified classification scheme guided by American Heart Association recommendations, based on the culmination of pathologic findings in more than 200 cases of sudden coronary death ( Box 1 ) . These defined morphologic criteria described for coronary arteries can be applied equally to carotid plaques and may serve as a unifying paradigm to understand the evolution of all atherosclerotic lesions, independent of the vascular bed.
Early nonsymptomatic carotid disease
Diffuse intimal thickening
Intimal xanthoma
Intermediate lesion
Pathologic intimal thickening
Progression of atherosclerosis leading to plaque enlargement
Plaque hemorrhage (± calcification)
Thin cap fibroatheroma (± calcification)
Lesions with thrombi
Plaque rupture with luminal thrombus
Plaque rupture with ulceration
Plaque rupture with organizing thrombus
Plaque erosion
Calcified nodule
Stable atherosclerotic plaque
Healed rupture/erosion
Fibrocalcific plaque
Total occlusion
Early nonsymptomatic disease
Intimal thickening and intimal xanthoma
Pre-existing, adaptive intimal thickening and the intimal xanthoma (“fatty streak”) are considered the earliest prelesional stage of the disease. Although some human lesions may begin as intimal xanthomas, considerable evidence suggests that the intimal thickening or mass lesion is most likely a precursor to symptomatic coronary disease in humans because these lesions are found in children in similar locations to those of advanced plaques in adults, whereas fatty streaks are know to regress . Histologically, intimal mass lesions consist mainly of smooth muscle cells and proteoglycan matrix, with a variable amount of lipid and little or no surrounding inflammation.
Pathologic intimal thickening
The transition from an early preatherosclerotic lesion to the more advanced fibroatheroma (defined by a true necrotic core) is marked by an intermediate morphology characterized by extracellular lipid pools within relatively acellular areas rich in proteoglycans . The lipid pools tend to develop at sites of adaptive intimal thickening and are located mostly in the deeper intimal layers close to the medial wall, whereas variable numbers of macrophages and T lymphocytes are seen outside the pool, mostly confined to the inner intima ( Fig. 1 ).
Advanced atherosclerosis
Fibrous cap atheroma
The first of the advanced coronary lesions, according to the American Heart Association, is the fibrous cap atheroma . The defining feature of this lesion is a lipid-rich necrotic core encapsulated by fibrous tissue ( Fig. 2 ). Fibrous cap atheroma may result in significant luminal narrowing and is also prone to the complications of surface disruption, thrombosis, and calcification. Knowledge of the origin and development of the core is fundamental in understanding the progression of atherosclerotic disease; the question of how lesions convert from lipid-rich pools within pathologic intimal thickening, to plaques with true necrosis (necrotic core), presents one of the most challenging issues in the field today.
Thin fibrous cap atheroma (vulnerable plaque)
Thin-cap fibroatheroma is characterized by a relatively large necrotic core representing about 25% of the plaque area, contained by a thin, fibrous cap defined at less than 65 μm, and heavily infiltrated by macrophages and some T-lymphocytes ( Fig. 3 ) . This well-characterized, ominous plaque is considered the prelude to rupture. The density of macrophages within the fibrous cap is generally very high, although in some cases macrophage infiltration may be less intense and occasionally absent. Because plaque rupture accounts for approximately 75% of thrombi in patients with sudden coronary death, early recognition of thin-cap fibroatheroma is paramount for the identification and treatment of potentially fatal lesions . The thinning or weakening of the fibrous cap leading to fissures and ruptures represents a common mechanism of fibrous cap disruption in coronary and carotid arteries . An interrupted fibrous cap allows circulating cellular and noncellular elements to come in direct contact with the highly thrombogenic components within the necrotic core, and is responsible for luminal thrombosis.
Fibrous cap thickness and lesion instability
It is becoming increasingly clear that fibrous cap thickness and plaque stress are predisposing elements to rupture. In a previous study of sudden coronary death victims, vulnerable plaque was defined histologically, based on a fibrous cap thickness less than 65 μm with regional infiltrates of macrophages (>25 per 0.3-mm–diameter field) . The critical thickness of 65 μm that renders a coronary lesion “vulnerable” is derived from a series of measurements at rupture sites where fibrous caps measured 23 ± 10 μm with 95% of caps 64 μm or less. A similar approach can be applied to carotid arteries with rupture. In a relatively large series of carotid plaques from the authors’ laboratory, the mean fibrous cap thickness was 72 ± 24 μm and vulnerable plaque thickness (95% of ruptured plaques) was identified as less than120 μm (R Virmani, unpublished data, 2007) (see Fig. 3 ). These values are remarkably within the range predicted by flow-plaque interaction models where a fibrous cap thickness of less than 0.1 mm is predicted to induce a large plaque stress that could precipitate rupture, even with an underlying 10% luminal stenosis . In another larger series of more than 412 symptomatic (major stroke or transient ischemic attack [TIA]) and asymptomatic patients who underwent carotid endarterectomy, vulnerable fibrous cap thickness (measured histologically) was defined at less than 165 microns, based on a mean (± SD) cap thickness of 70 ± 47 μm in ruptured plaques (A. Mauriello, G. Sangiorgi, R. Virmani, et al. unpublished data, 2007).
It is the authors’ experience that approximately one half of plaque ruptures occur toward the midportion of the cap, rather than the shoulder region, in autopsy plaques derived from sudden coronary deaths. Using a computational finite element model, Cheng and colleagues calculated the stress distribution in specific coronary artery lesions that caused lethal myocardial infarction and found the average maximum circumferential stress in ruptured plaques was 545 ± 160 kPa. Not all plaque ruptures occurred at the region of highest stress; 10 of 12 lethal lesions ruptured where calculated stress was not maximal, but was 300 kPa. A more precise explanation of why this occurs comes from a more recent study that suggests that minute calcifications of 10-μm –diameter found at sites of relatively high circumferential stress (>300 kPa) in the fibrous cap can intensify the stress levels to nearly 600 kPa when the cap thickness is less than 65 μm . Thus, the site of rupture depends on the relative location, concentrated circumferential stress, and the presence of microscopic calcific inclusions perhaps derived from apoptotic macrophages or smooth muscle cells are present . Collectively, these studies suggest that fibrous cap rupture is very likely a multifactorial event, unpredicted by stress factors or cap thickness alone.
In the coronary arteries of humans, plaque rupture generally occurs in lesions with less than 50% diameter stenosis. The authors’ laboratory and others suggest that plaque rupture is not a rare event in the evolution of coronary atherosclerosis . In this context, rupture of the lesion surface is followed by variable amounts of hemorrhage into the plaque, and luminal thrombosis, causing an often clinically silent progression of the disease.
Carotid plaques follow a similar pattern of disruption, with fibrous cap thinning and infiltration of macrophages (see Fig. 3 ). In a recent study, 47% of ruptured carotid plaque occurred in arterial segments with less than 70% cross-sectional luminal narrowing. Furthermore, a high prevalence of vulnerable plaques occurred in segments not significantly narrowed (80% of cases) (A. Mauriello, unpublished data, 2007). These data suggest that culprit lesions and their precursors occur more frequently in less severely narrowed vessels, and highlight the important tenet that plaques may progress to a substantial size through repeated ruptures before significant luminal stenosis occurs.
Lesions with thrombi
Plaque rupture with acute or early organizing luminal thrombi
Acute thrombosis is characterized predominantly by layered platelet aggregates with variable amounts of fibrin, red blood cells, and acute inflammatory cells. Over time, this provisional matrix heals through a concerted biologic process involving the infiltration of smooth muscle cells, accumulated extracellular matrix proteins (ie, proteoglycans and collagen), neovascularization, inflammation, and luminal surface re-endothelialization.
At least 75% to 80% of sudden coronary deaths show occlusive acute or organized thrombi, and death in the remaining cases is attributed to a “critical” greater than or equal to 75% cross-sectional stenosis in a lesion generally referred to as stable plaque . Although plaque rupture with luminal thrombosis is similarly considered to be the major cause of carotid stroke, thrombi occupying large portions of the lumen in these cases are unusual . The embolic nature of rupture in the carotid is uncharacteristic of coronary plaques because thrombotically active plaques are found in 74% of patients with ipsilateral stroke .
Carotid plaque rupture with ulceration
Most pathologists agree that plaque rupture with ulceration is the dominant mechanism that leads to thrombus formation in the carotid disease complicated by embolization and cerebral ischemic events . Plaque ulceration is defined as an excavated necrotic core underneath a ruptured fibrous cap, often with large areas of intraplaque hemorrhage and little or no luminal thrombus ( Fig. 4 ). If present, the thrombus is generally small and usually nonocclusive. In a recent large series of carotid endarterectomy specimens from symptomatic and asymptomatic patients that correlated risk factors to plaque morphology, ulcerated lesions were the most common finding, irrespective of clinical status (A. Mauriello, G. Sangiorgi, R. Virmani, et al., unpublished data, 2007). The larger vessel caliber and higher flow velocity of the carotid may explain the embolic nature of these plaques, where shear forces are greater than those of coronary circulation.
Plaque erosion with acute or early organizing luminal thrombi
Plaque erosion is the second-leading cause of acute coronary thrombosis in the coronary circulation. This lesion differs from that of rupture because there is an absence of fibrous cap disruption highlighted by a luminal surface rich in proteoglycans that lacks endothelium. The underlying lesion morphology also differs from rupture because it includes early lesions with pathologic intimal thickening or, to a lesser extent, fibroatheromas without extensive necrotic cores. Erosion is defined as an acute thrombus in direct contact with an intimal surface that lacks endothelial cell coverage. The morphologic characteristics of plaque erosion include an abundance of smooth muscle cells in a proteoglycan matrix and disruption of the surface endothelium without a prominent lipid core . Usually, few or no macrophages or T-lymphocytes are close to the lumen in plaque erosion. Erosions account for approximately 30% to 35% of cases of thrombotic sudden coronary death; they are more common in patients under the age of 50 and represent most of the acute coronary thrombi in premenopausal women .
Plaque erosion is an infrequent cause of acute thrombosis in the carotid artery, irrespective of symptoms . In the carotid vasculature, the incidence of erosion accounts for less than 10% of all luminal thrombi. It is suspected that the rarity of plaque erosions may be related to the higher flow rates, larger vessel caliber, or relative absence of vasospasm, because erosion in the coronary artery is attributed to episodic arterial contraction and loss of endothelial cells.
Nodular calcification
The “calcified nodule” represents the least frequent cause of luminal thrombus, accounting for 2% to 5% of coronary thrombi . This term is reserved for fibrocalcific plaques with little or no underlying necrotic core, with disruption at the luminal surface and thrombosis attributed to eruptive, dense, calcified nodules. These heavily calcified arteries often show large plates of calcified matrix with surrounding areas of fibrosis, inflammation, and neovascularization ( Fig. 5 ). Although the precise nature of this lesion remains incompletely understood, fragmentation of the calcified plates is believed to be the cause of the nodular calcification, where sometimes even bone formation is seen with interspersed fibrin. Although nodular calcification in deeper regions of the plaque is quite common in the carotid, its association with a luminal thrombus remains infrequent, constituting only 6% to 7% of thrombi (R Virmani, unpublished data, 2007).